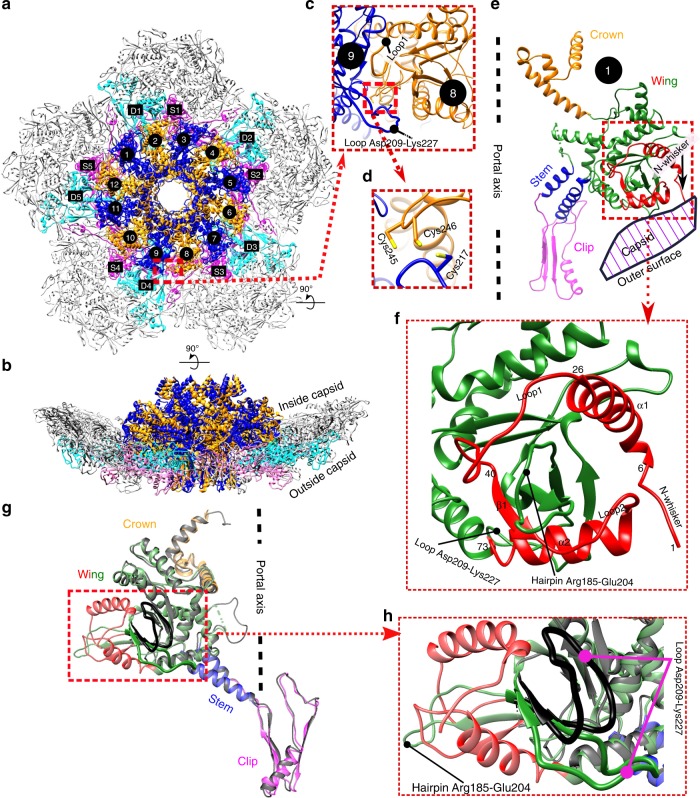
Fig. 1. In situ structure of the portal assembly and the surrounding capsomers.
a, b Overall structure, top and side views. The gp20 portal subunits are colored blue and orange and numbered 1 to 12. The S subunits (numbered S1 to S5) and D subunits (numbered D1 to D5) of the major capsid protein gp23* are colored magenta and cyan, respectively. c, d Inter-subunit interactions of the portal assembly. The enlarged inset shows the possible site for inter-subunit disulfide bonding. e, f Structure of gp20 subunit 1. The crown, stem, and clip domains are colored orange, blue, and magenta, respectively. The N-terminal 73 residues and other regions of the wing domain are colored red and green, respectively. The N-whisker region in e has been indicated by a black arrow. g, h Comparison of the in situ gp20 structure with the previously reported recombinantly expressed N-terminal deletion gp20 structure (PDB: 3JA7). The in situ gp20 structure (subunit 1) colored as in e is superimposed on the recombinant gp20 structure colored gray. The bent loop of the recombinant portal structure is shown in black.