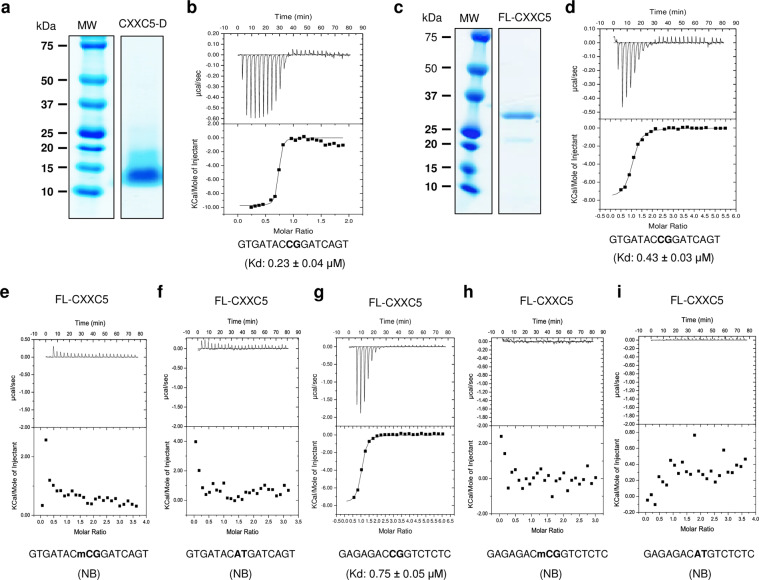
Figure 1.
Purification and interaction with DNA of the recombinant full-length CXXC5 (FL-CXXC5) and the CXXC domain (CXXC-D) proteins. CXXC-D (a) and FL-CXXC5 (c), expressed in bacteria and purified sequentially with ion exchange, heparin, and size-exclusion chromatographies, were loaded onto an SDS-PAGE gel (4–20% gradient) and stained with InstantBlue coomassie dye. “MW” indicates molecular masses in kDa. 10 µM CXXC-D (b) or FL-CXXC5 (d) was subjected to isothermal titration calorimetry (ITC) using a 300 µM double-stranded DNA fragment that bears a central unmethylated CG dinucleotide (5′-GTGATACCGGATCAGT-3′). (e,f) The binding of FL-CXXC5 was also assessed with ITC using a double-stranded DNA fragment with the central mCG or AT nucleotides embedded into the same surrounding DNA sequence. To ensure that sequences surrounding the central nucleotides have no effect on the ability of FL-CXXC5 to bind to DNA, a double-stranded DNA fragment with a distinct surrounding sequence (5′-GAGAGACxxGTCTCTC-3′) bearing the central CG (G), mCG (h) or AT (i) nucleotides was subjected to ITC. Results are the mean ± S.E. of two experiments. NB denotes no detectable binding.