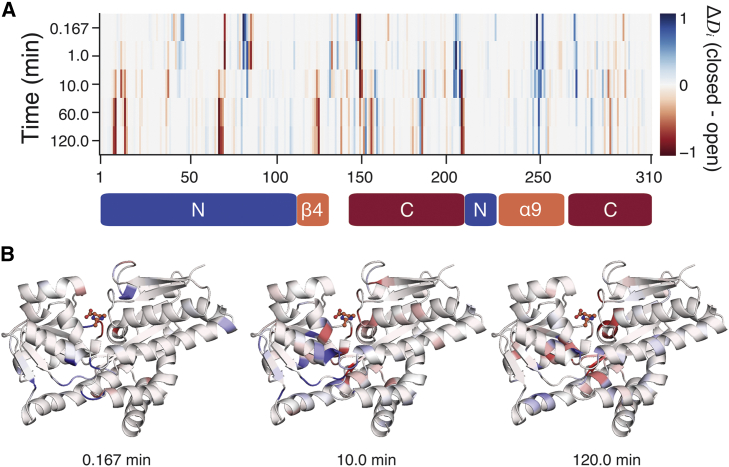
Figure 2.
Difference in predicted deuterated fractions between closed and open ensembles of TeaA. (A) By-residue ΔDi = Di,closed − Di,open for each time point is shown, where red indicates that a residue is more deuterated in the open conformation than in the closed, and blue indicates the opposite. Domain definitions are indicated using bars beneath the plot. (B) A representative closed structure of TeaA, colored by residue ΔDi at the 0.167, 10, and 120 min time points, is shown. The largest ΔDi-values are observed for residues either lining the central binding cleft or involved in the partial unfolding of helix α9 but are clearly not uniform across time points.