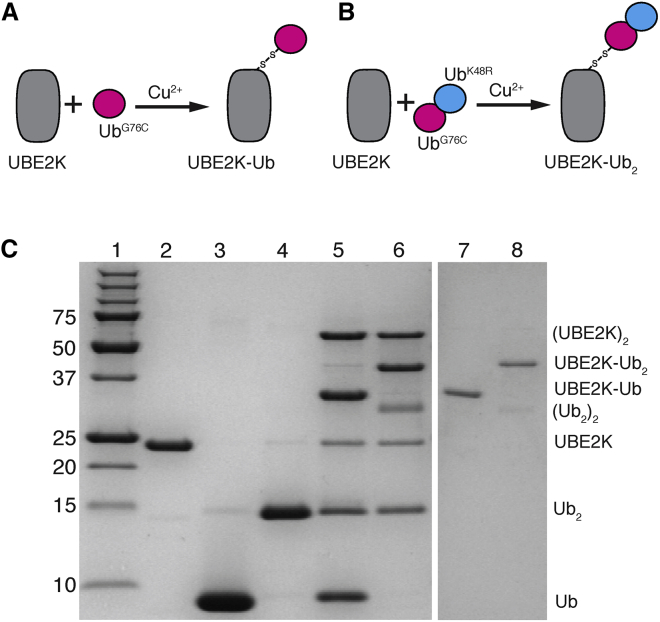
Figure 2.
Diagram showing the synthesis of disulfide-linked UBE2K-Ub and UBE2K-Ub2 complexes. Schematic representations showing the formation of the (A) UBE2K-Ub disulfide complex or (B) UBE2K-Ub2 disulfide complex using the E2 ubiquitin-conjugating enzyme UBE2K (gray) and UbG76C-UbK48R (magenta and cyan) components are given. Disulfide formation was accomplished using mild Cu2+ oxidation to link the C-terminal cysteine of UbG76C to the catalytic site (C92) of UBE2K to yield either UBE2K-UbG76C or UBE2K-UbG76C-UbK48R. (C) An SDS-PAGE gel showing the synthesis and purification of UBE2K-UbG76C or UBE2K-UbG76C-UbK48R is given. The gel shows molecular weight standards (lane 1), followed by purified UBE2K (lane 2), UbG76C (lane 3), and UbG76C-UbK48R (lane 4) proteins. The synthesis of UBE2K-UbG76C (lane 5) and UBE2K-UbG76C-UbK48R (lane 6) using Cu2+ oxidation is shown. Side products of these reactions include Ub2 and UBE2K2 as indicated. Size exclusion chromatography was used to obtain purified UBE2K-UbG76C (lane 7) and UBE2K-UbG76C-UbK48R (lane 8), as described in the Materials and Methods.