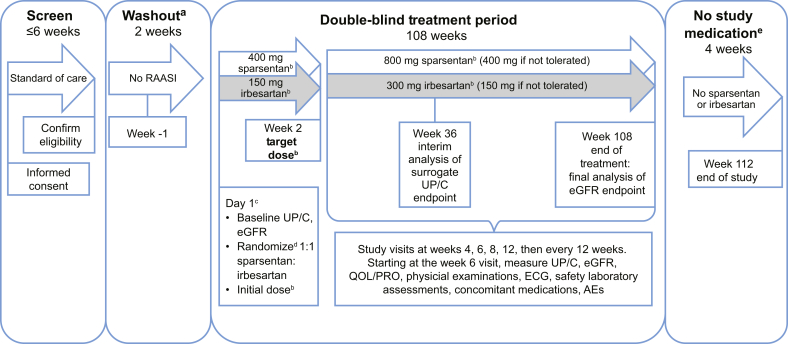
Figure 1.
A Randomized, Multicenter, Double-Blind, Parallel, Active-Control Study of the Effects of Sparsentan, a Dual Endothelin Receptor and Angiotensin Receptor Blocker, on Renal Outcomes in Patients With Primary FSGS study design. aFor patients who are undergoing washout from renin-angiotensin-aldosterone system inhibitor (RAASI). bPatients whose body weight is ≤50 kg at screening will receive half the otherwise specified doses of sparsentan or irbesartan (active control). Weight will be measured at each visit and the dose increased at the investigator’s discretion if the patient’s weight reaches >50 kg. cDay 1 events shown will occur in the order in which they are listed. dRandomization will be stratified by estimated glomerular filtration rate (eGFR) value (≥30 to <60 ml/min per 1.73 m2 and ≥60 ml/min per 1.73 m2 for all patients) and urinary protein-to-creatinine ratio (UP/C) (≤3.5 g/g and >3.5 g/g [patients ≥18 yr of age] or ≤2 g/g and >2 g/g [patients <18 yr of age]) at screening. eFollowing the 108-week blinded treatment period, treatment with study medication will be discontinued. At this time, the investigator should resume standard-of-care treatment, including treatment with RAASI (with the exception of irbesartan) provided there are no contraindications for their use. The investigator may make additional adjustments in antihypertensive medications as clinically indicated to adequately control the patient’s blood pressure. AEs, adverse events; ECG, electrocardiogram; PRO, patient-reported outcome; QOL, quality of life.