Abstract
Introduction
Nephrotic syndrome is associated with an increased risk of venous and arterial thromboembolism, which can be as high as 40% depending on the severity and underlying cause of nephrotic syndrome. The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend prophylactic anticoagulation only in idiopathic membranous nephropathy but acknowledge that existing data are limited and of low quality. There is a need for better identification of vulnerable patients in order to balance the risks of anticoagulation.
Methods
We undertook a systematic search of the topic in MEDLINE, EMBASE and COCHRANE databases, for relevant articles between 1990 and 2019.
Results
A total of 2381 articles were screened, with 51 full-text articles reviewed. In all, 28 articles were included in the final review.
Conclusion
We discuss the key questions of whom to anticoagulate, when to anticoagulate, and how to prophylactically anticoagulate adults with nephrotic syndrome. Using available evidence, we expand upon current KDIGO guidelines and construct a clinical algorithm to aid decision making for prophylactic anticoagulation in nephrotic syndrome.
Keywords: anticoagulation, arterial thromboembolism, nephrotic syndrome, prophylaxis, venous thromboembolism
Graphical abstract
Nephrotic syndrome (NS) is a manifestation of glomerular disease characterized by >3.5 g/d of proteinuria, hypoalbuminemia, dyslipidemia, and edema. Primary glomerular pathologies frequently associated with NS include, but are not limited to, membranous glomerulonephritis (MGN), minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), and membranoproliferative glomerulonephritis (MPGN). Important secondary causes include diabetic nephropathy and amyloid disease. One serious clinical implication of NS is the development of a hypercoagulable state, placing patients at increased risk for venous thromboembolism (VTE) and arterial thromboembolism (ATE).1 In some instances, thromboembolic disease is the initial presenting symptom, leading to the subsequent discovery of NS.2, 3, 4
Pathophysiology
Hypercoagulability reflects an imbalance in coagulation homeostasis created by urinary losses of anti-thrombotic factors and increased hepatic synthesis of pro-thrombotic factors. There are reduced levels of the natural anticoagulant antithrombin III, and higher levels of clotting factors V, VIII, and fibrinogen in patients with NS.5,6 Protein C and S levels may also be reduced, but this is a less consistent finding.7
Abnormalities are also found in platelet function. Albumin loss results in increased free arachidonic acid and formation of thromboxane A2, leading to platelet aggregation, which is further accentuated by hypertriglyceridemia, another feature of NS.8,9 Other contributing factors are the potential pro-coagulopathic role of corticosteroids, a staple of many glomerulonephritis treatment regimens, and volume contraction in NS patients with diuretic use.
In-depth analyses on the mechanisms of hypercoagulability in NS have been extensively covered in the literature and are beyond the scope of our review.
Risk of Thromboembolism
Bellomo and Atkins found venous thromboembolic events to occur in 40% of patients with membranous nephropathy.10 Deep vein thrombosis is said to develop in approximately 15% of patients with NS, and renal vein thrombosis had been reported to develop in 25% to 30% of patients with NS, with the greatest risk seen in MGN (37%), MPGN (26%), and MCD (24%).11
In a retrospective cohort study of 298 patients with NS, Mahmoodi et al. examined the rates of objectively verified thromboembolic events and reported an annual incidence of 1% and 1.5% for VTE and ATE in this population, respectively. This study verifies high absolute risks of symptomatic VTE and ATE that were remarkably elevated within the first 6 months of diagnosis.1 For comparison, the rate of VTE in the general population is estimated to be 1 to 2 per 1000 persons per year.12
Similar findings were reported by Kayali et al., in a retrospective analysis of patients discharged from U.S. hospitals, identifying those with NS and deep vein thrombosis (DVT), pulmonary embolism (PE), or renal vein thrombosis (RVT). Approximately 1.5% and 0.5% of admitted patients with NS between 1979 and 2005 in this study experienced DVT and PE respectively, with those between 18 and 39 years of age at greatest risk.13
The risk of VTE and ATE, however, varies among patients with NS, and these events are associated with high rates of morbidity and mortality, with a 6% and 12% incidence of death at 30 days following DVT and PE, respectively.14,15 There is therefore a need for accurate identification of those individuals who are most vulnerable, in order to balance the risks of anticoagulation.
Aims
Unfortunately, firm evidence on the optimal population to treat, and regimen to treat with has remained elusive, as high-quality clinical trials are difficult to carry out. It has been estimated 972 patients are required to adequately power a clinical trial because of the low overall frequency of events.16 The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend prophylactic anticoagulation in idiopathic membranous glomerulonephritis only, but acknowledge that existing data are limited and of low quality.17
In this context, we undertook a systematic review to address the key questions of who, when, how, and for how long to prophylactically anticoagulate adult patients with nephrotic syndrome. Using the evidence, we build on the current KDIGO recommendations and construct a clinical algorithm that may aid clinicians in their decision making for prophylactic anticoagulation in a patient with NS.
Methods
We undertook a systematic search of MEDLINE, EMBASE and COCHRANE databases from the period of 1990 to August 2019.
Search Strategy
We searched within MEDLINE and EMBASE databases using keywords detailed below. Medical Subject Headings (MeSH) were used where available with all subheadings included. Keywords were allocated to group 1 or group 2 for the purposes of the search.
Group 1 search terms were “nephrotic syndrome,” “membranous glomerulonephritis,” “minimal change glomerulonephritis,” “minimal change disease,”,“lipoid nephrosis,” and “focal sclerosing glomerulosclerosis.”
Group 2 search terms were “thromboembolism,” “venous thromboembolism,” “arterial thromboembolism,” “arterial occlusive diseases,” “thrombosis,” “venous thrombosis,” “pulmonary embolism,” “deep vein thrombosis,” “anticoagulation,” “anticoagulants,” “warfarin,” “apixaban,” “rivaroxaban,” “dabigatran,” “edoxaban,” and “heparin.”
Group 1 search terms were combined individually with group 2 search terms using the Boolean operator “AND.” The subsequent pairings were combined with “OR” operator to generate a single pool of results.
We searched the COCHRANE database for the following combinations: “nephrotic syndrome AND anticoagulation,” “nephrotic syndrome AND thrombosis,” “nephrotic syndrome AND thromboembolism,” “nephrotic syndrome AND warfarin,” “nephrotic syndrome AND heparin,” “membranous glomerulonephritis AND anticoagulation,” “minimal change disease AND anticoagulation,” and “focal sclerosing glomerulonephritis AND anticoagulation.”
The pooled results from each database were combined and duplicates removed. Articles were then screened for relevance.
Inclusion and Exclusion Criteria
Articles were included if they pertained to the epidemiology of thromboembolic disease in NS, risk factors associated with thromboembolic disease in NS, prophylaxis of thromboembolic disease in NS, or treatment of thromboembolic disease in NS. We included only articles available in English. Articles were excluded if they were clearly related to other subject matter. Articles were also excluded if they were review or opinion articles, did not clearly outline treatment parameters, or did not provide adequate follow-up or description of outcomes. We also excluded articles examining only pediatric populations.
Algorithm Formulation
The rationale for our algorithm to approach prophylactic anticoagulation in NS patients can be found in the Discussion.
Results
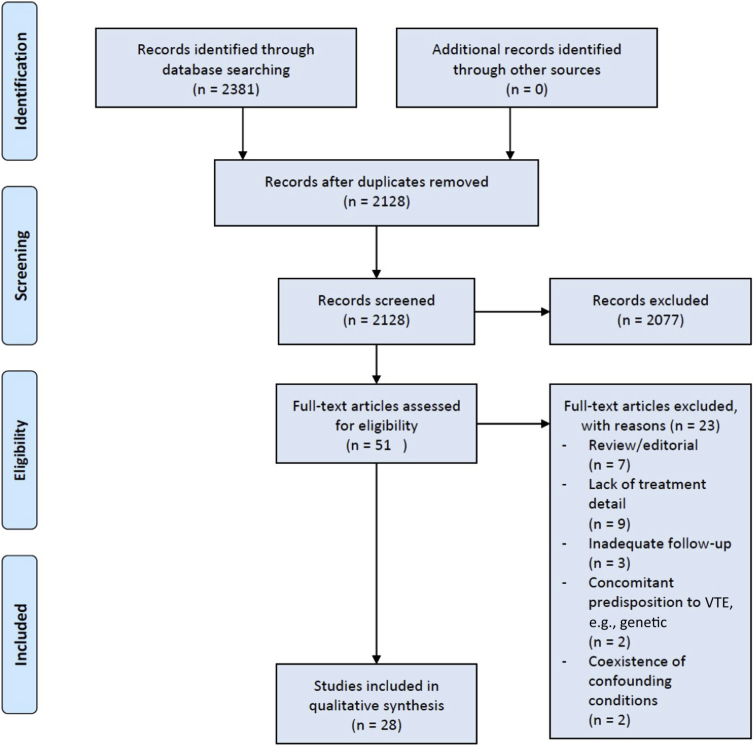
Our search strategy returned 564 articles on MEDLINE, 1777 articles on EMBASE, and 40 articles on the COCHRANE database. After de-duplication, 2128 articles remained, which were screened. After screening, 51 full-text articles were identified and reviewed as potentially suitable, with 28 articles being included in the final review (Figure 1).
Figure 1.
Search strategy and results. VTE, venous thromboembolism.
Articles pertaining to epidemiology and risk factors for thromboembolism in NS are summarized in Table 1,13,18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 and articles examining prophylaxis or treatment of thromboembolism in NS are summarized in Table 230, 31, 32, 33, 34 and Table 3.35, 36, 37, 38, 39, 40, 41, 42, 43
Table 1.
Studies assessing epidemiology and risk factors for ATE and VTE in nephrotic syndrome
| Authors | Year | Modality | Sample size | Histology | Primary outcome(s) | Results and key observations |
|---|---|---|---|---|---|---|
| Barbour et al.18 | 2012 | Retrospective cohort study | 1313 | MGN (30.1%) FSGS (28.2%) IgAN (41.7%) |
VTE (DVT, PE, RVT, other) | 44 Patients (3.4%) experienced VTE Histologic subtype, particularly MGN, was independent risk factor for VTE |
| Fenton et al.19 | 2018 | Retrospective cohort study | 78 | MCD | VTE | 9 Patients (12%) developed VTE |
| Gyamlani et al.20 | 2017 | Retrospective cohort study | 7037 | MCD, FSGS, MPGN, MGN Diabetic nephropathy Unclassified |
VTE (DVT, PE, RVT) | 158 VTEs over follow-up period 3.17 Events per 1000 patient years Proportionate increase in event rate with declining SA SA threshold as high as 40 g/l |
| Harza et al.21 | 2013 | Prospective cohort study | 191 | MGN (29%) FSGS (25%) IgAN (18%) MPGN (15%) MCD (13%) |
VTE (DVT, PE, RVT) | 23 Patients (12%) developed VTEs Most VTEs occurred in first 6 months (65.2%) MGN + MPGN highest risk subtypes |
| Kayali et al.13 | 2008 | Retrospective cohort study | 925,000 | Unspecified | VTE (DVT, PE, RVT) | 1.5% Had DVT, 0.5% had PE, RVT less common Relative risk of DVT in NS was 6.81 in age group 18−39 yr |
| Kumar et al.22 | 2012 | Retrospective cohort study | 101 | MGN (77% primary, 23% secondary) | VTE (DVT, PE), stroke, ATE | 15 Patients (19.2%) with primary MGN experienced VTE Risk was highest in first 6 mo Risk factors: severe hypoalbuminemia, hypercholesterolemia, and proteinuria |
| Li et al.23 | 2012 | Prospective study | 100 | MGN | VTE (PE, RVT) | 36 Patients (36%) experienced VTE |
| Li et al.24 | 2016 | Prospective cohort study | 120 | FSGS | VTE | 12 Patients (10%) experienced VTE |
| Lionaki et al.25 | 2012 | Retrospective cohort study | 898 | MGN | VTE | 65 Patients (7%) experienced VTE Hypoalbuminemia (SA <28 g/l) most significant risk factor for VTE risk |
| Maas et al.26 | 2017 | Retrospective cohort study | 125 | MCD | VTE, ATE | 11 Patients (9%) experienced VTE or ATE |
| Mahmoodi et al.1 | 2008 | Retrospective cohort study | 298 | MGN (24%) MCD (16%) FSGS (12%) MPGN (9%) Diabetic nephropathy (11%) |
VTE, ATE | Annual incidence of VTE/ATE was 1.02%/1.48% respectively in patients with NS Risk was highest in first 6 mo |
| Waldman et al.27 | 2007 | Retrospective cohort study | 95 | MCD | VTE, ATE | 4 Patients (4.2%) developed thrombosis in follow-up period |
| Zhang et al.28 | 2014 | Prospective cohort study | 512 | MGN (35.7%) FSGS (7%) SLE (11.5%) MPGN (2.9%) MCD (2.2%) IgAN (4.9%) Not specified (17.4%) |
PE, RVT | 180 Patients (35%) had PE and/or RVT MGN and increasing age were independent predictors of PE and/or RVT |
| Zou et al.29 | 2018 | Retrospective cohort study | 766 | MGN | VTE (DVT, RVT, PE) ATE (stroke, PVD) |
53 Patients (6.9%) experienced VTE 71 Patients (9.3%) experienced ATE Low SA was independent risk factor Risk highest in first 6 mo |
ATE, arterial thromboembolic event; DVT, deep vein thrombosis, FSGS, focal sclerosing glomerulosclerosis; IgAN, IgA nephropathy; MCD, minimal change disease; MGN, membranous glomerulonephritis; MPGN, membranoproliferative glomerulonephritis; PE, pulmonary embolism; PVD, peripheral vascular disease; RVT, renal vein thrombosis; SA, serum albumin; SLE, systemic lupus erythematosus; VTE, venous thromboembolic event.
Histological subtypes are included where available and important study outcomes are listed.
Table 2.
Studies assessing use of warfarin or heparin as prophylaxis or treatment of thromboembolism in nephrotic syndrome
| Authors | Year | Modality | Sample size | Histology | Intervention | Prophylaxis versus treatment | Results and key observations |
|---|---|---|---|---|---|---|---|
| Kelddal et al.30 | 2019 | Retrospective cohort study | 79 | MCD (44.3%) MGN (24.1%) FSGS (8.9%) Other (22.8%) |
Warfarin alone, warfarin with bridging LMWH, high/low-dose LMWH versus none | Prophylaxis | More thromboembolism events in nonprophylaxis group. Prophylaxis may increase bleeding risk, particularly if concurrent aspirin. |
| Medjeral-Thomas et al.31 | 2014 | Retrospective cohort study | 143 | MGN (40.6%) MCD (31.4%) FSGS (28.0) |
SA <20 g/l: LMWH 20 mg or warfarin (INR, 1.5−2.5) SA 20−30g /l: aspirin 75 mg |
Prophylaxis | No VTEs in patients with established prophylaxis >1 wk 2 VTEs occurred in patients within 1 wk of commencing prophylaxis |
| Rostoker et al.32 | 1995 | Prospective pilot study | 55 | MGN (29%) MCD (27%) FSGS (23.6%) SLE (3.6%) Other (16.4%) |
LMWH 40 mg | Prophylaxis | LMWH was effective and safe with low incidence of bleeding |
| Wang et al.33 | 2011 | Case report | 1 | MCD | LMWH | Treatment | Successful treatment of mesenteric vein thrombosis and portal vein thrombosis |
| Yang et al.34 | 2002 | Case report | 1 | MCD | LMWH | Treatment | Successful treatment of renal vein thrombosis |
FSGS, focal sclerosing glomerulosclerosis; INR, international normalized ratio; LMWH, low−molecular-weight heparin; MCD, minimal change disease; MGN, membranous glomerulonephritis; SA, serum albumin; SLE, systemic lupus erythematosus; VTE, venous thromboembolic event.
Histological subtypes are included where available, and important study outcomes are listed.
Table 3.
Studies assessing use of direct-acting anticoagulants as prophylaxis or treatment of thromboembolism in nephrotic syndrome
| Authors | Year | Modality | Sample size | Histology | Intervention | Prophylaxis versus treatment | Results and key observations |
|---|---|---|---|---|---|---|---|
| Basu et al.35 | 2015 | Case report | 1 | SLE-related NS | Rivaroxaban | Treatment and prophylaxis | Failure of rivaroxaban in prophylaxis New splenic infarcts |
| Dupree et al.36 | 2014 | Case report | 1 | Not specified | Rivaroxaban | Treatment | Recurrent VTE while on warfarin Successful use of rivaroxaban for 6 mo without recurrent VTE |
| Han et al.37 | 2017 | Case report | 1 | MGN | Rivaroxaban | Treatment | New-onset renal vein thrombosis on rivaroxaban for pulmonary embolism Successful treatment with warfarin |
| Li et al.38 | 2019 | Case report | 1 | Not specified | Rivaroxaban | Treatment | Poor response to rivaroxaban Good response to warfarin with bridging heparin |
| Reynolds et al.39 | 2019 | Case report | 1 | MGN | Apixaban | Treatment | Recurrent VTE despite therapeutic apixaban |
| Sasaki et al.40 | 2014 | Case report | 1 | MGN | Dabigatran | Treatment | Successful treatment of carotid thromboembolism with dabigatran |
| Sexton et al.41 | 2018 | Case report | 2 | MCD MGN |
Apixaban | Prophylaxis | No thrombosis in follow-up periods of 8 wk and 3 mo |
| Shimada et al.42 | 2017 | Case report | 1 | Not specified | Edoxaban | Treatment | Recurrence of PE on warfarin Edoxaban used to good efficacy |
| Zhang et al.43 | 2018 | Randomized trial | 16 | MCD (43.8%) MGN (25%) FSGS (12.5%) Other (12.5%) LN (6.25%) |
LMWH vs. rivaroxaban | Treatment | 7 of 8 Patients achieved thrombus dissolution Rivaroxaban a safe and effective single-agent treatment option |
FSGS, focal sclerosing glomerulosclerosis; LMWH, low−molecular-weight heparin; LN, lupus nephritis; MCD, minimal change disease; MGN, membranous glomerulonephritis; SLE, systemic lupus erythematosus; VTE, venous thromboembolic event.
Histological subtypes are included where available and important study outcomes listed.
Discussion
The “Who”
Histological Subtype of Nephrotic Syndrome
The histological subtype of NS is an important factor in assessing the risk of thromboembolism. Membranous glomerulonephritis appears to confer an especially high risk of thrombosis among the causes of NS. Barbour et al. analyzed patients from the Toronto Glomerulonephritis Registry, identifying 1313 patients with MGN, FSGS, and IgA nephropathy (IgAN). After adjustment for key variables including malignancy, histological subtype was observed to be an independent predictor for VTE, with MGN carrying a hazard ratio of 10.8 when compared against IgAN.18
Other studies have yielded similar findings: MGN was reported to be the most common histological subtype associated with PE in a prospective study of 512 consecutive NS patients,21,28 whereas others have simply reported very high rates of thromboembolic events in patients with MGN-related NS, with one series finding that 36% of patients with MGN experienced a VTE.22,23,25,29
The other histological subtypes of NS also share the risk of thrombosis, although the risk does not appear to be as pronounced. Studies on the clinical outcomes of patients with MCD and FSGS have demonstrated high thrombotic rates during follow-up,19,24,26,27 whereas others have found high risks of VTE and ATE in NS across a range of histological subtypes.1,44 One study found the risk of thrombosis in MCD to be higher than MGN in their analysis.16
The observation of greater thrombotic risk in MGN compared to other subtypes, even after correcting for serum albumin and proteinuria, is not well understood. One hypothesis is a dissimilarity in the way in which MGN affects the coagulation cascade compared to other subtypes. Huang et al. conducted a comparison of coagulation parameters using blood tests and thromboelastography in 101 MGN patients, 61 MCD patients, and 20 healthy controls. They observed that the R value, which is the time taken for the first evidence of clot to be observed, was significantly lower in MGN patients compared with MCD patients and controls.45 An association between MGN and genetic predispositions to thromboembolism, such as Factor V Leiden, have also been postulated.46,47
Summary
The presence of NS, regardless of histological subtype, appears to confer a clinically relevant potential for thrombotic complications, occurring at a significantly higher rate than in the general population. The risk appears to be accentuated if the underlying disease is MGN, although the pathophysiology behind this observation is not entirely clear. Current KDIGO guidelines suggest anticoagulation only for idiopathic MGN; however, available evidence suggests that perhaps anticoagulation can be considered in all patients with NS.
The “When”
Serum Albumin
Serum albumin has been observed to be a strong predictor of VTE in NS.20,25,27,48,49 The KDIGO guidelines reflect this in their recommendation for anticoagulation in patients with MGN when serum albumin falls to less than 25 g/l in the presence of other thrombotic risk factors.17
The severity of hypoalbuminemia is thought to be a surrogate marker for the degree of imbalance in pro-thrombotic and anti-thrombotic factors, translating into elevated thrombotic risk. Lionaki et al. reported a serum albumin threshold of 28 g/l, below which there was a 3.9-fold increase in VTE risk, rising to 5.8-fold when serum albumin was less than 22 g/l in MGN patients.25 Gyamlani et al., in a retrospective analysis of 7,037 U.S. veterans, found that thrombotic risk rose proportionately with declining serum albumin, and even mild reductions in serum albumin (30−39.9 g/l) conferred increased risk. The highest-risk group were those with serum albumin less than 25 g/l, with an event rate of 8.5 per 1000 patient years.20
One limitation of the current data, however, is the uncertainty as to whether serum albumin thresholds established in these studies are applicable across all histological subtypes of NS. The available studies have either included MGN patients only, or were unable to analyze the subgroups of NS separately when looking at serum albumin thresholds (Table 1).
Summary
Most forms of NS appear to carry some clinically significant risk of thrombosis, and anticoagulation should be considered in the setting of hypoalbuminemia. The serum albumin threshold below which to commence anticoagulation is not clearly established, although most studies concur that the level is between 20 and 30 g/l. This will need to be balanced with the patient’s risk of bleeding. If proceeding with anticoagulation, it should be commenced as soon as it is safe, as the risk of thrombosis has consistently been found to be highest in the first 6 months of diagnosis.1,21,29
Risk of Bleeding
The decision to prescribe anticoagulation cannot occur without a simultaneous assessment of the patient’s risk of bleeding. As randomized trials with sufficient power are difficult to perform, much of our risk assessment strategies are derived from the non-NS population.
The closest we have to a decision-making framework currently is a clinical tool derived from a study published in 2014. Lee et al. used a Markov decision process to quantify the benefits of anticoagulation versus the risk of major bleeding in patients with idiopathic MGN.50 The study population was taken from the 2012 study of 898 patients by Lionaki et al.,25 and bleeding risk was represented by the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) score. The authors concluded that patients derive variable benefit from prophylactic anticoagulation, depending on their risk of bleeding and serum albumin level. For instance, if the patient was at low risk for bleeding, there was a reasonable risk−benefit ratio from anticoagulation even at serum albumin thresholds as high as 30 g/l. Conversely, for those at high risk for bleeding, the risk−benefit ratio does not become favorable at any serum albumin level. Their clinical tool (https://www.med.unc.edu/gntools/) is currently available on the webpage hosted by the University of North Carolina School of Medicine.51
There are, however, limitations to the clinical tool developed by Lee et al. The most obvious is that it is applicable only to patients with MGN, and so the risk−benefit ratio of anticoagulation in patients with other forms of NS remains unclear. The ATRIA score may also not be the best predictor of bleeding. The HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly [> 65 years], Drugs/alcohol concomitantly) scoring system52 has repeatedly been found to be a better predictor of bleeding compared to other scoring systems, including ATRIA, across multiple comparisons.53, 54, 55, 56, 57, 58
Perhaps most important is the uncertainty of the application of any of these bleeding risk assessment tools to a NS patient. The physiology of anticoagulation in nephrotic syndrome is complex and not fully elucidated. A recent study by Kawai et al. found that serum albumin levels of less than 36 g/l were associated with more major bleeding events and a trend toward supratherapeutic international normalized ratio (INR), although the study was not targeted to the specific mechanism of hypoalbuminemia encountered in NS.59
Summary
Anticoagulation does not appear to be beneficial for patients with high bleeding risk scores, regardless of serum albumin. There are no validation studies for bleeding risk assessment scores in the NS population. Acknowledging this, the HAS-BLED scoring system has better correlation with bleeding risk in the general population compared to other tools. Clinicians should be mindful that the risk of bleeding estimated by these scoring systems may not be accurate, particularly in severe hypoalbuminemia, thus mandating close clinical follow-up.
The “How”
Warfarin and Heparin
Warfarin, a vitamin K antagonist and staple anticoagulant across many indications, is the recommended agent in the KDIGO 2012 guidelines for VTE prophylaxis in MGN-related NS. Low-molecular-weight heparin (LMWH) is given as an alternative for prophylaxis, but uncertainty exists in dosing, as heparin’s target of action, antithrombin III, is actively lost in urine in NS, potentially conferring heparin resistance.60 For treatment of documented arterial or venous thromboembolism, the guidelines suggest full-dose anticoagulation with either LMWH or warfarin.17
Unfortunately, there is little evidence available in the literature on the optimal prophylactic agent or its dosing, owing to a lack of randomized trials. The available evidence consists primarily of case studies or retrospective cohort studies that represent local treatment practices and protocols rather than a universally agreed-upon treatment strategy (Table 2).
Rostoker et al., in 1995, presented a prospective pilot study in 55 patients with various NS subtypes. Patients were given LMWH (enoxaparin) at 40 mg for variable time periods, and this was found to be safe, well tolerated, and effective for thrombosis prevention.32 Medjeral-Thomas et al. carried out a single-center retrospective analysis in 143 patients with NS between 2006 and 2011. Those with serum albumin <20 g/l received enoxaparin 20 mg (or low-dose warfarin; INR, 1.5−2.5) and those with serum albumin 20 to 30 g/l received daily aspirin 75 mg. There were no VTEs in patients who had received prophylaxis for at least 1 week, and the 2 VTEs that were observed occurred within the first week of treatment.31 The authors concluded that a regimen of prophylactic antiplatelet or anticoagulant therapy appears to be effective in preventing VTE in nephrotic syndrome, with relatively few hemorrhagic complications.
More recently, Kelddal et al. retrospectively examined 79 patients with NS, 44 of whom received some form of prophylactic anticoagulation, compared with 35 who did not. The prophylaxis regimens varied, from warfarin alone, warfarin with bridging LMWH, or low/high-dose LMWH alone. The authors observed that there were significantly more thromboembolic events in the nontreated group, and that major bleeding only occurred in those who had concurrent aspirin treatment for other reasons.30
With regard to dosing, the American College of Chest Physicians Clinical Guidelines for Prevention of Thrombosis advise that the optimal INR range for prevention of thrombosis is 2.0 to 3.0.61 Although it is worth noting that this INR recommendation was not developed for an NS population, in the absence of contrary evidence or validation studies, it is a reasonable target for warfarin dosing. LMWH dosing is less certain, as there is the choice between prophylactic or therapeutic dosing. The majority of studies using heparin as prophylaxis have opted for prophylactic over therapeutic dosing.
Overall, it is difficult to draw conclusions as to whether these varied prophylactic regimens were truly effective. First, the studies were limited by their observational design. In addition, the number of patients in each study were relatively few, meaning that thrombotic events may simply have not occurred in the intervention groups purely by chance. It can also be difficult to account for changes in thrombotic risk during follow-up; for instance, as patients enter remission with treatment, their risk may naturally decline.
Summary
Available studies suggest that prophylactic anticoagulation is beneficial for thrombosis prevention in at-risk NS patients, although major caveats apply, including the lack of randomized controlled trials. The efficacy of warfarin versus heparin has not been compared directly by any study. Heparin, despite the theoretical possibility of resistance from antithrombin III loss, has been used successfully for both prophylaxis and treatment.33,34 Low-dose aspirin was used for low-risk patients in one study.31 If proceeding with prophylaxis, the choice of agent should be guided by patient and clinical factors, such as ease of use and access, patient preference, and feasibility of monitoring requirements.
Direct Oral Anticoagulants
Direct oral anticoagulants (DOACs) were first introduced in 2008, and work by directly inhibiting factor Xa (rivaroxaban, apixaban, edoxaban) or thrombin (dabigatran, argatroban) in the coagulation cascade. Clinical applications for these agents are widening, and they are first-line options for conditions such as nonvalvular atrial fibrillation, DVT, and PE.62 DOACs are increasingly favored over warfarin for their rapid onset of activity, lack of monitoring requirement, and reduced bleeding profile; however, their role in NS is not well defined.
The evidence for use of DOACs in NS is extremely limited, consisting mainly of case reports and one small randomized trial, with the majority of studies focused on treatment rather than prophylaxis of thrombosis. We include studies using DOACs for treatment of thrombosis in our discussion, as it illustrates the complexities and our lack of understanding of the coagulation system in NS.
The use of DOACs for treatment of thromboembolism have produced mixed results. Some authors have reported failure of DOACs,35,37, 38, 39 requiring subsequent reversion to traditional agents, whereas others have reported success where traditional anticoagulants have fallen short or were poorly tolerated.36,40,42 The only randomized trial data are those from a small pilot study by Zhang et al., in which 16 patients were allocated to receive either rivaroxaban or LMWH for treatment of VTE. Nearly all patients achieved the endpoint of thrombus dissolution, and the authors concluded that rivaroxaban was an effective alternative option for treatment of NS-related thrombosis.43
Evidence for DOACs as prophylaxis is limited to only one case report detailing the successful use of apixaban as prophylaxis in 2 patients with MGN and MCD41 (Table 3).
The other major consideration for use of DOACs is safety. DOACs are currently not approved for use in patients with significant renal impairment (creatinine clearance <30 mL/min per 1.73 m2), although there is mounting evidence to suggest that these agents can be safe in the renal impairment population, so their use may gradually become more widespread.63,64
Summary
Evidence for the prophylactic use of DOACs in the setting of NS is extremely limited, and the majority of evidence pertains to treatment of VTE rather than prophylaxis. Overall results are mixed. Given that much of the pharmacokinetics and pharmacodynamics of these agents in NS remain uncertain, the use of DOACs as first-line agents for prophylaxis and treatment of ATE/VTE in NS should be carefully considered. DOACs may be reasonable alternatives to warfarin and LMWH in the event of the failure or intolerability of these agents, allowing for renal function.
Why Does Anticoagulation Fail?
The literature demonstrates both success and failure with contemporary anticoagulants, particularly DOACs, but the reasons for failure are poorly understood. Beyond common reasons for medication failure such as medication nonadherence, potential contributors in the setting of NS are alterations in the pharmacokinetics of anticoagulation agents, our clinical expertise with adjusting anticoagulation dose, and the heterogeneity of changes to the coagulation system.
Pharmacokinetics of Anticoagulants
Some authors have hypothesized that the pharmacokinetics and pharmacodynamics of anticoagulants are altered by hypoalbuminemia,35,39 which merits discussion. The mechanisms of action, bioavailability, volume of distribution, and degree of renal clearance for warfarin, heparin, rivaroxaban, apixaban, and dabigatran are summarized in Table 4.64, 65, 66, 67, 68, 69, 70, 71
Table 4.
Pharmacokinetics of currently available anticoagulants
| Pharmacological property | Warfarin65 | Low−molecular-weight heparin64, 66, 67, 68, 69 | Rivaroxaban64, 70 | Apixaban64 | Dabigatran71 |
|---|---|---|---|---|---|
| Mechanism of action |
Vitamin K antagonist |
Potentiation of antithrombin III | Direct Xa inhibitor | Direct Xa inhibitor | Direct thrombin (factor II) inhibitor |
| Site of action | Factors II, VII, IX, X inhibition | Factors IIa, IXa, Xa, XIa, XIIa inhibition | Factor Xa inhibition | Factor Xa inhibition | Thrombin, prothrombin inhibition |
| Bioavailability (oral) |
99%−100% |
90% (subcutaneous) | 80%−100% | 66% | 6%−7% |
| Protein binding |
99% |
Variablea | 95% | 87% | 35% |
| Volume of distributionb |
10 L |
7 L | 50 L | 21 L | 60−70 L |
| Renal clearance |
Very low |
Primarily renal clearance | 33% (66% via liver metabolism) | 27% | 85% |
Protein binding of heparin is variable and dependent on molecular chain length. Increased protein binding reduces efficacy.
Volume of distribution based on 70-kg man with 42 L total body water. Volume of distribution <10 L implies restriction of drug to intravascular fluid. Volume of distribution >42 L implies distribution to tissues in the body.
Warfarin, apixaban, and rivaroxaban are highly protein bound. The implications are whether high urinary protein loss translates to excess free drug and increased bleeding risk, or, conversely, whether protein-bound drug is rapidly lost in urine, reducing efficacy. Alternatively, NS may not affect the performance of the drug at all, as suggested by Gugler et al. in a pharmacokinetic study of 3 drugs with varying levels of protein binding. In the highly protein-bound drug (diphenylhydantoin), the percentage of free drug doubled in NS patients because of lower serum protein; however, this was counterbalanced by a lower steady-state concentration due to increased clearance, resulting in no overall net difference.72 Keller et al. supported this observation and suggested that in NS and other renal disease, free-drug concentration is a more reliable measure of drug efficacy than total plasma concentration.73
In 1986, Ganeval et al. reported on the pharmacokinetics of warfarin in 11 NS patients compared with 11 normal controls. Warfarin levels in urine were undetectable in the majority of patients, despite being protein bound; and although the blood concentration of free-drug doubled, the overall plasma clearance increased 3-fold, leading to a significantly shorter half-life.74 These results suggest the effect of NS on the pharmacokinetics of warfarin and other protein-bound anticoagulants, may not be readily predictable, particularly if other factors such as oral bioavailability, changes in volume of distribution, and drug−drug interactions are taken into consideration.
Clinical Experience and Scope in Anticoagulation Dosing
Perhaps the greater success in the literature for the use warfarin and heparin in NS compared with DOACs is due to our ability to readily measure their efficacy (INR and anti-Xa activity) and make appropriate adjustments to dose, thus avoiding toxicity and maintaining efficacy.
Drug levels or measures of efficacy are performed far less often for DOACs, as one of their key strengths is their predictable pharmacokinetic profile. This creates the potential for subtherapeutic or supratherapeutic drug activity to go undetected. This concept is illustrated in a case report of a patient with MCD who required much higher doses of apixaban (10−15 mg twice a day) to maintain therapeutic drug levels,75 yet these doses are not routinely prescribed in practice for fear of toxicity and bleeding. This is further compounded by the reduced scope for adjusting the dose of DOACs because of lack of clinical experience and rigid dosing regimens.
The ongoing study of the pharmacokinetics of apixaban in NS, measuring anti-Xa activity at numerous time points, will hopefully further our understanding of this area and allow greater insights into how to dose these agents (ClinicalTrials.gov ID: NCT0259532).
Heterogeneity of Changes in the Coagulation System
There is potentially considerable heterogeneity within alterations to the coagulation system, creating different risk profiles within each individual patient that cannot be readily accounted for. Kerlin et al., collating observations from other studies, demonstrated that changes in pro-coagulant, anti-coagulant, and fibrinolytic factors in NS can range from being increased, to unchanged, to decreased.76 Although some of the discrepancies in observations can likely be attributed to varying study methodologies, it should be acknowledged that the precise mechanism by which NS interacts with these factors is unknown, and is not simply a function of molecular size.
Summary
Much work is needed to understand the complex pharmacokinetics and pharmacodynamics of anticoagulants in the setting of NS. Although there is deep clinical experience in the monitoring and dose adjustment of warfarin and heparin, the same experience is lacking in DOACs, as they are relatively new and have rigid dosing regimens in place. The predictable pharmacokinetic profile of DOACs in the general population, which usually allows for no regular drug monitoring, may need to be reconsidered in the NS. Should DOACs be incorporated into future guidelines for anticoagulation in NS, some form of drug efficacy and toxicity monitoring may be required.
Duration of Treatment
Optimal duration of treatment is poorly established. The KDIGO guidelines suggest continuation of prophylaxis while the patient remains nephrotic (serum albumin <30 g/l), presumably as the pathophysiological processes predisposing to thrombus risk theoretically remain. To date, there are no studies specifically addressing this question, which is not surprising, given the overall level of data available.
In addition to the challenges posed by low event rates and patient numbers, any prospective study hoping to answer this question will need to address the issue of how to account for relapses in the underlying condition. For instance, in the event of thrombotic events occurring after cessation of anticoagulation, the question will arise as to whether this was due to a relapse of NS, or whether the anticoagulation was ceased “too early.”
Algorithm: Individualized Approach to Prophylactic Anticoagulation in Nephrotic Syndrome
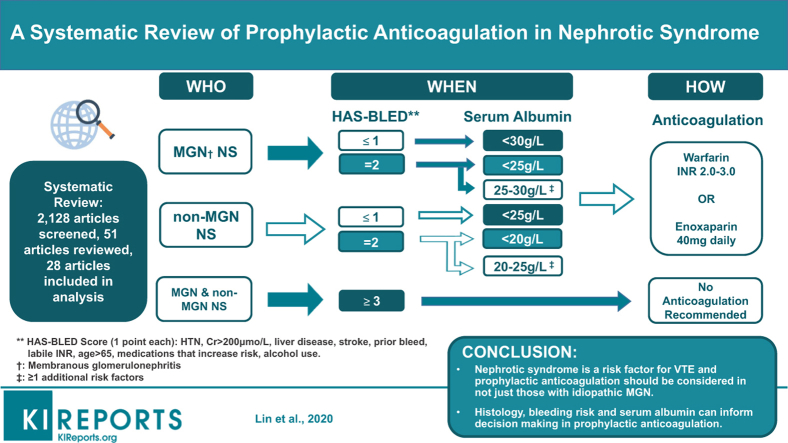
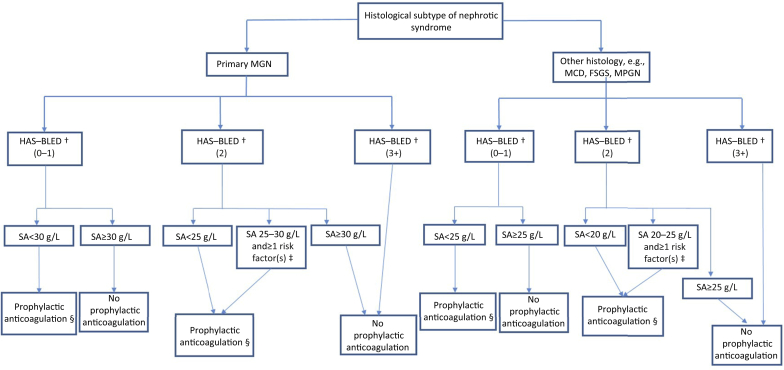
Using available evidence in combination with existing KDIGO guidelines, we present an algorithm to aid decision making on VTE prophylaxis in NS (Figure 2). We suggest considering the following 3 key patient parameters when making this decision.
Figure 2.
Algorithm for suggested approach to thromboembolism prophylaxis in nephrotic syndrome patients. †HAS-BLED scores for bleeding risk: 0−1, low risk; 2, moderate risk; 3−5, high risk; 5+, very high risk (see Supplementary Table S1). ‡Additional risk factors: proteinuria >10 g/d, body mass index >35 kg/m2, documented genetic predisposition to venous thromboembolism (VTE), prolonged immobilization, recent abdominal or orthopedic surgery, New York Heart Association Class III to IV congestive heart failure. §Prophylactic anticoagulation first-line therapy: warfarin (international normalized ratio [INR], 2.0−3.0) or enoxaparin 40 mg daily. FSGS, focal segmental glomerulosclerosis; HAS-BLED, Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (> 65 years), Drugs/alcohol concomitantly); MCD, minimal change disease; MGN, membranous glomerulonephritis; MPGN, membranoproliferative glomerulonephritis; SA, serum albumin.
Histology
The first distinction is the presence of MGN as opposed to other histological subtypes. The literature suggests that MGN generally confers a higher risk of thrombosis and thus lowers our threshold to commence prophylaxis.
Risk of Bleeding
We use the HAS-BLED scoring system to stratify patients into low-, moderate-, or high-risk bleeding groups (Supplementary Table S1). Drawing from the work of Lee et al., anticoagulation should not be pursued in patients at highest risk for bleeding (HAS-BLED score of ≥3) regardless of histological subtype or serum albumin level.50
Serum Albumin
For MGN, if bleeding risk is low, we selected a higher serum albumin threshold of 30 g/l below which to consider anticoagulation, based on the results of Lionaki et al. and Gyamlani et al.20,25 If the patient is at moderate risk for bleeding (HAS-BLED score of 2), we suggest that the serum albumin threshold be reduced to <25 g/l, which is in line with current KDIGO guidelines. Patients with a serum albumin level between 25 and 30 g/l should be considered for anticoagulation if there are one or more additional risk factors of heavy proteinuria >10 g/d, high personal or genetic risk of VTE, morbid obesity, advanced heart failure, immobilization, or recent abdominal/orthopedic surgery.
For non-MGN subtypes, the algorithm is similar, but the serum albumin below which anticoagulation should be considered is lower (25 g/l for patients with low bleeding risk and 20 g/l for those with moderate bleeding risk), reflecting the lower risk of thrombosis in NS associated with non-MGN subtypes.
Conclusions
Many uncertainties remain regarding anticoagulation in NS. Available evidence suggests that patients with MGN have the highest rates of thrombosis, although other NS subtypes also carry clinically significant risk. Limiting anticoagulation prophylaxis to only those patients with MGN may therefore need to be reconsidered.
Susceptibility to thrombosis escalates with declining serum albumin levels, but bleeding is the main consideration against initiating anticoagulation. A combination of all 3 aspects, which we have incorporated into a suggested algorithm, should inform clinical decision making.
Clinical trials to definitively answer these questions are difficult to perform, and we are likely to be dependent on meta-analyses and systematic reviews for future guidance. For this reason, further studies in this area should strive to maintain uniform treatment regimens to allow gradual accumulation of data, and we hope that our algorithm can contribute to this goal. Pharmacokinetic and pharmacodynamic studies on the actions of anticoagulants in NS will further our understanding.
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary Material
Table S1. HAS-BLED system for risk of bleeding with anticoagulation.
Supplementary Material
References
- 1.Mahmoodi B.K., ten Kate M.K., Waanders F. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation. 2008;117:224–230. doi: 10.1161/CIRCULATIONAHA.107.716951. [DOI] [PubMed] [Google Scholar]
- 2.Khanna A. Undiagnosed and unsuspected nephrotic syndrome in a young adult presenting as submassive pulmonary embolism. Chest. 2016;149:A506. [Google Scholar]
- 3.Qureshi M., Alabi F., Christian F., Romero C. The forgotten urinalysis: an integral part of unmasking thrombophilia. J Community Hosp Intern Med Perspect. 2019;9:40–44. doi: 10.1080/20009666.2018.1562854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pallavi R., Sunggeun L., Baumstein D. Stroke in a young woman as a presenting manifestation of membranous nephropathy. Am J Ther. 2016;23:e950–e954. doi: 10.1097/MJT.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 5.Kauffmann R.H., Veltkamp J.J., Van Tilburg N.H., Van Es L.A. Acquired antithrombin III deficiency and thrombosis in the nephrotic syndrome. Am J Med. 1978;65:607–613. doi: 10.1016/0002-9343(78)90848-3. [DOI] [PubMed] [Google Scholar]
- 6.Rabelink T.J., Zwaginga J.J., Koomans H.A., Sixma J.J. Thrombosis and hemostasis in renal disease. Kidney Int. 1994;46:287–296. doi: 10.1038/ki.1994.274. [DOI] [PubMed] [Google Scholar]
- 7.Cosio F.G., Harker C., Batard M.A. Plasma concentrations of the natural anticoagulants protein C and protein S in patients with proteinuria. J Lab Clin Med. 1985;106:218–222. [PubMed] [Google Scholar]
- 8.Jackson C.A., Greaves M., Patterson A.D. Relationship between platelet aggregation, thromboxane synthesis and albumin concentration in nephrotic syndrome. Br J Haematol. 1982;52:69–77. doi: 10.1111/j.1365-2141.1982.tb03862.x. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho A.C., Colman R.W., Lees R.S. Platelet function in hyperlipoproteinemia. N Engl J Med. 1974;290:434–438. doi: 10.1056/NEJM197402212900805. [DOI] [PubMed] [Google Scholar]
- 10.Bellomo R., Atkins R.C. Membranous nephropathy and thromboembolism: is prophylactic anticoagulation warranted? Nephron. 1993;63:249–254. doi: 10.1159/000187205. [DOI] [PubMed] [Google Scholar]
- 11.Glassock R.J. Prophylactic anticoagulation in nephrotic syndrome: a clinical conundrum. J Am Soc Nephrol. 2007;18:2221–2225. doi: 10.1681/ASN.2006111300. [DOI] [PubMed] [Google Scholar]
- 12.Scheres L.J.J., Lijfering W.M., Cannegieter S.C. Current and future burden of venous thrombosis: not simply predictable. Res Pract Thromb Haemost. 2018;2:199–208. doi: 10.1002/rth2.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kayali F., Najjar R., Aswad F. Venous thromboembolism in patients hospitalized with nephrotic syndrome. Am J Med. 2008;121:226–230. doi: 10.1016/j.amjmed.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 14.White R.H. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 suppl 1):I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 15.Ageno W., Squizzato A., Garcia D., Imberti D. Epidemiology and risk factors of venous thromboembolism. Semin Thromb Hemost. 2006;32:651–658. doi: 10.1055/s-2006-951293. [DOI] [PubMed] [Google Scholar]
- 16.Rankin A.J., McQuarrie E.P., Fox J.G., Scottish Renal Biopsy R. Venous thromboembolism in primary nephrotic syndrome–is the risk high enough to justify prophylactic anticoagulation? Nephron. 2017;135:39–45. doi: 10.1159/000448628. [DOI] [PubMed] [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2 [Google Scholar]
- 18.Barbour S.J., Greenwald A., Djurdjev O. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 2012;81:190–195. doi: 10.1038/ki.2011.312. [DOI] [PubMed] [Google Scholar]
- 19.Fenton A., Smith S.W., Hewins P. Adult minimal-change disease: observational data from a UK centre on patient characteristics, therapies, and outcomes. BMC Nephrol. 2018;19:207. doi: 10.1186/s12882-018-0999-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gyamlani G., Molnar M.Z., Lu J.L. Association of serum albumin level and venous thromboembolic events in a large cohort of patients with nephrotic syndrome. Nephrol Dial Transplant. 2017;32:157–164. doi: 10.1093/ndt/gfw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harza M., Ismail G., Mitroi G. Histological diagnosis and risk of renal vein thrombosis, and other thrombotic complications in primitive nephrotic syndrome. Rom J Morphol Embryol. 2013;54:555–560. [PubMed] [Google Scholar]
- 22.Kumar S., Chapagain A., Nitsch D., Yaqoob M.M. Proteinuria and hypoalbuminemia are risk factors for thromboembolic events in patients with idiopathic membranous nephropathy: an observational study. BMC Nephrol. 2012;13:107. doi: 10.1186/1471-2369-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S.-J., Guo J.-Z., Zuo K. Thromboembolic complications in membranous nephropathy patients with nephrotic syndrome–a prospective study. Thromb Res. 2012;130:501–505. doi: 10.1016/j.thromres.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Li S.J., Tu Y.M., Zhou C.S. Risk factors of venous thromboembolism in focal segmental glomerulosclerosis with nephrotic syndrome. Clin Exp Nephrol. 2016;20:212–217. doi: 10.1007/s10157-015-1149-4. [DOI] [PubMed] [Google Scholar]
- 25.Lionaki S., Derebail V.K., Hogan S.L. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol. 2012;7:43–51. doi: 10.2215/CJN.04250511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maas R.J., Deegens J.K., Beukhof J.R. The clinical course of minimal change nephrotic syndrome with onset in adulthood or late adolescence: a case series. Am J Kidney Dis. 2017;69:637–646. doi: 10.1053/j.ajkd.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 27.Waldman M., Crew R.J., Valeri A. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007;2:445–453. doi: 10.2215/CJN.03531006. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L.J., Zhang Z., Li S.J. Pulmonary embolism and renal vein thrombosis in patients with nephrotic syndrome: prospective evaluation of prevalence and risk factors with CT. Radiology. 2014;273:897–906. doi: 10.1148/radiol.14140121. [DOI] [PubMed] [Google Scholar]
- 29.Zou P.M., Li H., Cai J.F. A cohort study of incidences and risk factors for thromboembolic events in patients with idiopathic membranous nephropathy. Chin Med Sci J. 2018;33:91–99. doi: 10.24920/11809. [DOI] [PubMed] [Google Scholar]
- 30.Kelddal S., Nykjær K.M., Gregersen J.W., Birn H. Prophylactic anticoagulation in nephrotic syndrome prevents thromboembolic complications. BMC Nephrol. 2019;20:139. doi: 10.1186/s12882-019-1336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medjeral-Thomas N., Ziaj S., Condon M. Retrospective analysis of a novel regimen for the prevention of venous thromboembolism in nephrotic syndrome. Clin J Am Soc Nephrol. 2014;9:478–483. doi: 10.2215/CJN.07190713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rostoker G., Durand-Zaleski I., Petit-Phar M. Prevention of thrombotic complications of the nephrotic syndrome by the low-molecular-weight heparin enoxaparin. Nephron. 1995;69:20–28. doi: 10.1159/000188355. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y.C., Chuang F.R., Lee W.C. Low-molecular-weight heparin successfully used to treat a nephrotic patient complicated by superior mesenteric vein thrombosis and portal vein thrombosis. Med Princip Pract. 2011;20:196–199. doi: 10.1159/000319925. [DOI] [PubMed] [Google Scholar]
- 34.Yang S.H., Lee C.H., Ko S.F. The successful treatment of renal-vein thrombosis by low-molecular-weight heparin in a steroid-sensitive nephrotic patient. Nephrol Dial Transplant. 2002;17:2017–2019. doi: 10.1093/ndt/17.11.2017. [DOI] [PubMed] [Google Scholar]
- 35.Basu A., Jain S., Patel D. Failure of anticoagulation in a case of nephrotic syndrome with recurrent thromboembolism. Chest. 2015;148:983A. [Google Scholar]
- 36.Dupree L.H., Reddy P. Use of rivaroxaban in a patient with history of nephrotic syndrome and hypercoagulability. Ann Pharmacother. 2014;48:1655–1658. doi: 10.1177/1060028014549349. [DOI] [PubMed] [Google Scholar]
- 37.Han T., Han C., Thet Z. Warfarin vs new oral anticoagulant in primary adult nephrotic syndrome associated venous thormboembolism. Nephrology. 2017;22(suppl 3):64. [Google Scholar]
- 38.Li Y., Chen Y., Qi X. Poor response to rivaroxaban in nephrotic syndrome with acute deep vein thrombosis: a case report. Medicine. 2019;98 doi: 10.1097/MD.0000000000016585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds M.L., Nachman P.H., Mooberry M.J. Recurrent venous thromboembolism in primary membranous nephropathy despite direct Xa inhibitor therapy. J Nephrol. 2019;32:669–672. doi: 10.1007/s40620-018-0552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki Y., Raita Y., Uehara G. Carotid thromboembolism associated with nephrotic syndrome treated with dabigatran. Case Rep Nephrol Urol. 2014;4:42–52. doi: 10.1159/000362162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sexton D.J., de Freitas D.G., Little M.A. Direct-acting oral anticoagulants as prophylaxis against thromboembolism in the nephrotic syndrome. Kidney Int Rep. 2018;3:784–793. doi: 10.1016/j.ekir.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimada Y., Nagaba Y., Nagaba H. Edoxaban was effective for treating renal vein thrombosis in a patient with nephrotic syndrome. Intern Med. 2017;56:2307–2310. doi: 10.2169/internalmedicine.8742-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L., Zhang H., Zhang J. Rivaroxaban for the treatment of venous thromboembolism in patients with nephrotic syndrome and low AT-III: a pilot study. Exp Ther Med. 2018;15:739–744. doi: 10.3892/etm.2017.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rankin A., McQuarrie E., Fox J.G. Is the risk of venous thromboembolism overstated in primary nephrotic syndrome? Nephrol Dial Transplant. 2015;3:iii109. [Google Scholar]
- 45.Huang M.J., Wei R.B., Wang Z.C. Mechanisms of hypercoagulability in nephrotic syndrome associated with membranous nephropathy as assessed by thromboelastography. Thromb Res. 2015;136:663–668. doi: 10.1016/j.thromres.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 46.Elinav E., Rubinger D., Hiller N. Renal vein thrombosis and membranous glomerulopathy in a patient homozygote for factor V Leiden mutation: a mere coincidence? Thromb Haemost. 2006;95:740–743. [PubMed] [Google Scholar]
- 47.Sahin M., Ozkurt S., Degirmenci N.A. Assessment of genetic risk factors for thromboembolic complications in adults with idiopathic nephrotic syndrome. Clin Nephrol. 2013;79:454–462. doi: 10.5414/CN107863. [DOI] [PubMed] [Google Scholar]
- 48.Kuhlmann U., Steurer J., Bollinger A. [Incidence and clinical significance of thromboses and thrombo-embolic complications in nephrotic syndrome patients] Schweiz Med Wochenschr. 1981;111:1034–1040. [PubMed] [Google Scholar]
- 49.Ismail G., Mircescu G., Ditoiu A.V. Risk factors for predicting venous thromboembolism in patients with nephrotic syndrome: focus on haemostasis-related parameters. Int. Urol. Nephrol. 2014;46:787–792. doi: 10.1007/s11255-013-0574-0. [DOI] [PubMed] [Google Scholar]
- 50.Lee T., Biddle A.K., Lionaki S. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int. 2014;85:1412–1420. doi: 10.1038/ki.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nachman P., Reich H.N. Prophylactic anticoagulation in patients with membranous nephropathy: a decision analysis. University of North Carolina School of Medicine website. https://www.med.unc.edu/gntools/index.html Available at:
- 52.Pisters R., Lane D.A., Nieuwlaat R. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 53.Apostolakis S., Lane D.A., Guo Y. Performance of the HEMORR(2)HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol. 2012;60:861–867. doi: 10.1016/j.jacc.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 54.Apostolakis S., Lane D.A., Guo Y. Performance of the HEMORR 2 HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in nonwarfarin anticoagulated atrial fibrillation patients. J Am Coll Cardiol. 2013;61:386–387. doi: 10.1016/j.jacc.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Roldan V., Marin F., Fernandez H. Predictive value of the HAS-BLED and ATRIA bleeding scores for the risk of serious bleeding in a "real-world" population with atrial fibrillation receiving anticoagulant therapy. Chest. 2013;143:179–184. doi: 10.1378/chest.12-0608. [DOI] [PubMed] [Google Scholar]
- 56.Klok F.A., Kooiman J., Huisman M.V. Predicting anticoagulant-related bleeding in patients with venous thromboembolism: a clinically oriented review. Eur Respir J. 2015;45:201–210. doi: 10.1183/09031936.00040714. [DOI] [PubMed] [Google Scholar]
- 57.Senoo K., Proietti M., Lane D.A., Lip G.Y. Evaluation of the HAS-BLED, ATRIA, and ORBIT bleeding risk scores in patients with atrial fibrillation taking warfarin. Am J Med. 2016;129:600–607. doi: 10.1016/j.amjmed.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Fauchier L., Chaize G., Gaudin A.F. Predictive ability of HAS-BLED, HEMORR2HAGES, and ATRIA bleeding risk scores in patients with atrial fibrillation. A French nationwide cross-sectional study. Int J Cardiol. 2016;217:85–91. doi: 10.1016/j.ijcard.2016.04.173. [DOI] [PubMed] [Google Scholar]
- 59.Kawai M., Harada M., Motoike Y. Impact of serum albumin levels on supratherapeutic PT-INR control and bleeding risk in atrial fibrillation patients on warfarin: a prospective cohort study. Int J Cardiol Heart Vasc. 2019;22:111–116. doi: 10.1016/j.ijcha.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Durrani J., Malik F., Ali N., Jafri S.I.M. To be or not to be a case of heparin resistance. J Community Hospital Intern Med Perspect. 2018;8:145–148. doi: 10.1080/20009666.2018.1466599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guyatt G.H., Akl E.A., Crowther M. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):7s–47s. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vílchez J.A., Gallego P., Lip G.Y.H. Safety of new oral anticoagulant drugs: a perspective. Ther Adv Drug Saf. 2014;5:8–20. doi: 10.1177/2042098613507945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mavrakanas T.A., Samer C.F., Nessim S.J., Frisch G., Lipman M.L. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28:2241–2248. doi: 10.1681/ASN.2016090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MIMS [database online]. St Leonards, NSW: Australia: MIMS Australia Pty Ltd. http://www.mims.com.au/ Available at:
- 65.Hirsh J., Warkentin T.E., Shaughnessy S.G. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1 suppl):64s–94s. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- 66.Manson L., Weitz J.I., Podor T.J. The variable anticoagulant response to unfractionated heparin in vivo reflects binding to plasma proteins rather than clearance. J Lab Clin Med. 1997;130:649–655. doi: 10.1016/s0022-2143(97)90115-3. [DOI] [PubMed] [Google Scholar]
- 67.Young E., Cosmi B., Weitz J., Hirsh J. Comparison of the non-specific binding of unfractionated heparin and low molecular weight heparin (Enoxaparin) to plasma proteins. Thromb Haemost. 1993;70:625–630. [PubMed] [Google Scholar]
- 68.Young E., Wells P., Holloway S. Ex-vivo and in-vitro evidence that low molecular weight heparins exhibit less binding to plasma proteins than unfractionated heparin. Thromb Haemost. 1994;71:300–304. [PubMed] [Google Scholar]
- 69.Smythe M.A., Priziola J., Dobesh P.P. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:165–186. doi: 10.1007/s11239-015-1315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol. 2012;26:27–32. doi: 10.1111/j.1472-8206.2011.00981.x. [DOI] [PubMed] [Google Scholar]
- 71.Feldberg J., Patel P., Farrell A. A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol Dial Transplant. 2019;34:265–277. doi: 10.1093/ndt/gfy031. [DOI] [PubMed] [Google Scholar]
- 72.Gugler R., Shoeman D.W., Huffman D.H. Pharmacokinetics of drugs in patients with the nephrotic syndrome. J Clin Invest. 1975;55:1182–1189. doi: 10.1172/JCI108035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keller F., Maiga M., Neumayer H.H. Pharmacokinetic effects of altered plasma protein binding of drugs in renal disease. Eur J Drug Metab Pharmacokinet. 1984;9:275–282. doi: 10.1007/BF03189651. [DOI] [PubMed] [Google Scholar]
- 74.Ganeval D., Fischer A.M., Barre J. Pharmacokinetics of warfarin in the nephrotic syndrome and effect on vitamin K-dependent clotting factors. Clin Nephrol. 1986;25:75–80. [PubMed] [Google Scholar]
- 75.Wharin S., Gooding R. A case series of patients treated with escalated doses of doacs. Br J Haematol. 2018;181(suppl 1):142. [Google Scholar]
- 76.Kerlin B.A., Ayoob R., Smoyer W.E. Epidemiology and pathophysiology of nephrotic syndrome–associated thromboembolic disease. Clin J Am Soc Nephrol. 2012;7:513–520. doi: 10.2215/CJN.10131011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.