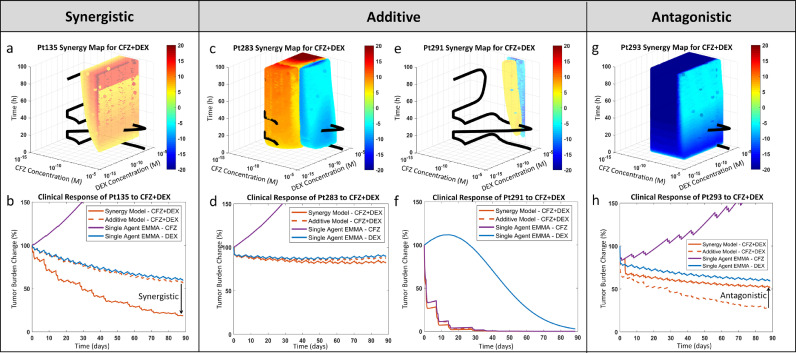
Fig. 4.
Interpatient heterogeneity in combination effect and clinical relevance of synergy. a, Synergy map for Pt135's response to carfilzomib and dexamethasone: The theoretical additive response is estimated from the single agents' models (EMMA) and subtracted from the combination model (SAM) estimated ex vivo response for the first 96 hours over a wide range of concentrations/concentration ratios. The difference is presented as a heat map, where a benefit over additive (synergy) is indicated as ‘hot’ (yellow-red) and a loss in percent viability is marked as ‘cold’ (cyan-blue). The synergy map also features the pharmacokinetic curve of the standard of care therapeutic regimen for this combination. The relative residence period of the pharmacokinetic curve in the hot/cold regions qualitatively shows the extent of clinically relevant synergistic/antagonistic effect. b, Pt135's predicted clinical response to carfilzomib and dexamethasone: The two single agent clinical response simulations (via EMMA), along with additive and combination response simulations (via SAM), are shown. The residence of the pharmacokinetic curve in the synergistic region is reflected in the clinical prediction. This analysis is repeated in c and d for Pt283, e and f for Pt291, and g and h for Pt293. These four patients were classified as early relapse/refractory at the time of their biopsy. In spite of their similar classification, the synergy maps and the clinical response simulations show significant variation. Abbreviations: CFZ, carfilzomib; DEX, dexamethasone; Pt, patient; h, hours; M, molar.