Abstract
Dual-specificity phosphatase-1 (DUSP1/MKP1) plays a key role in controlling various physiological and pathological phenomena, including tumor metastasis and invasion. However, the role of MKP1 in tumorigenesis is controversial. We showed that the expression of MKP1 in hepatocellular carcinoma (HCC) is significantly downregulated, and MKP1 is an independent predictor of poor prognosis. In in vitro and in vivo studies, we showed that MKP1 significantly inhibits the invasion and metastasis of HCC cells. Additionally, we found that low MKP1 expression is associated with the expression of ROCK2, which plays an important role in HCC. Our data suggest that MKP1 is crucial for ROCK2-mediated metastasis and invasion. Interestingly, we demonstrated that ROCK2 has opposite effects on protein and mRNA levels of MKP1, as it decreases the expression at the protein level and increases the expression at the mRNA level. We also identified the mechanism responsible for this incongruency; ROCK2 activates ERK1/2-ATF2 signaling, which leads to the increased mRNA expression of MKP1. At the same time, ROCK2 promotes the ubiquitin-mediated degradation of MKP1 by activating ERK1/2, therefore promoting the metastasis of HCC. In conclusion, our data provide new evidence for the biological and clinical significance of MKP1 as a potential biomarker. We demonstrate that ROCK2 disturbs the protein and mRNA expression of MKP1 in human HCC progression.
Keywords: Hepatocellular carcinoma, MKP1, metastasis, ROCK2, ERK1/2, ATF2, ubiquitination
Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, the fifth most common cancer worldwide, and the second leading cause of cancer death in men. HCC accounts for 70-85% of the total cancer burden [1]. Although some progress has been made in its clinical detection and treatment in recent years, the metastasis and recurrence rates of HCC after radical resection are still high [2]. Therefore, it is urgent to find new diagnostic markers and therapeutic targets for this disease.
Dual-specificity phosphatase-1 (DUSP1/MKP1) is a threonine-tyrosine bispecific phosphatase [3]. Despite recent research efforts, the role of MKP1 in tumorigenesis is still controversial [4,5]. Some studies have shown that MKP1 is highly expressed in some tumours, and may promote the occurrence of prostate cancer by inhibiting Fas/FasL-induced apoptosis [6]. Knockout of MKP1 can reduce the incidence of cancer [7]. Contrarily, a different study showed that MKP1 expression is low in certain tumor types compared to adjacent non-tumour control tissues, where MKP1 inhibits tumorigenesis [8,9]. However, the expression and role of MKP1 in HCC are largely unknown.
Rho-related coiled-coil containing protein kinase 2 (ROCK2) is one of the most studied targets for small GTPase Rho [10]. ROCK2 is a key signaling molecule in the Rho/ROCK signalling pathway, which regulates gene expression by regulating the activity or phosphorylation of target proteins [11]. Several reports have suggested that the increased expression of ROCK2 is associated with the occurrence and development of many human cancers, including HCC [12,13]. Overexpression of ROCK2 in HCC is significantly associated with the formation of microsatellites [14]. Recently, ROCK2 has been reported to be critical for cancer cell migration and invasion [15]. Specifically, it has been shown that, in HCC, stable knockout of Rock2 significantly reduces the migration and invasion of HCC cells in vitro and in vivo [16]. Interestingly, we previously found that the protein level of MPK1 is upregulated in ROCK2 knockdown HCC cells by analyzing tandem mass tags and mass spectrometry data (Table S1). Thus, we presumed that ROCK2 could affect the metastasis and invasion in HCC by modulating MPK1 expression.
In this study, we found that MKP1 could serve as a prognostic factor and potential therapeutic target for HCC. Additionally, we investigated the mechanism of action of MPK1 in HCC and explored how MPK1 expression is regulated by ROCK2.
Materials and methods
Patients and samples
From December 2012 to January 2018, 132 HCC specimens were collected from 132 patients who underwent hepatectomy at the Jiangxi Cancer Hospital (China). All specimens obtained during the operation were frozen immediately in liquid nitrogen and stored at -80°C for further analysis. Pathologists confirmed the nature of tumours and adjacent normal tissues. Informed consent was obtained for each patient, and the study was approved by the Ethical Committee of the Jiangxi Cancer Hospital.
Cell culture
The human HCC cell lines Huh-7 (CVCL_0336), MHCC97H (CVCL_4927) and Hep3B (CVCL_0326) were purchased from the Shanghai Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences (Shanghai, China). The HCCLM3 (CVCL_6832) cell line is a derivative of the MHCC97H cell line, purchased from the China Center for Type Culture Collection (CCTCC). All cell lines had been authenticated using STR profiling by the FuHeng Cell Center (Shanghai, China) within the last three years. All experiments were performed with mycoplasma-free cells.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from tissues and cultured cells using TRIzol (Invitrogen, USA) according to the manufacturer’s instructions. PrimeScript RT kit (Invitrogen) was used to retro transcribe the RNA. For qRT-PCR analysis, cDNA was amplified using a SYBR green PCR kit (Applied Biosystems, Carlsbad, CA, USA). GAPDH was used as internal control.
Animal studies
BALB/c nude mice (4 weeks old, female) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. Cells (1×107 cells in 100 ml phosphate buffer) were injected into the caudal vein of anesthetized nude mice (6 mice per group). Six weeks following tumor injection, mice were euthanized with lung tissues collected for haematoxylin-eosin staining and analyses. All animal work was approved by the Animal Experimental Ethics Committee of the Jiangxi Cancer Hospital and carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals (8th edition).
Cell migration and invasion assays
The migration/invasion of cancer cells was routinely examined at laboratory. For the invasion assays, the polycarbonate membranes in the upper chambers were precoated with manufacturer.
IHC staining and immunofluorescence (IF) assays
HCC samples and adjacent non-tumour tissues were fixed, embedded, sectioned, and deparaffinised. For IHC staining, non-specific antibody binding sites in the sections were blocked using a serum-free protein block buffer (DAKO, CA, USA) for 30 min; sections were then incubated with an anti-MKP1 antibody (1:200, Abcam). For IF assays, the cells (2×103) were grown on slides. Non-specific antibody binding sites were blocked with 5% BSA at room temperature 20-25°C (68-77°F) and the cells were then stained with anti-MKP1 (1:100), anti-ROCK2 (1:500), anti-ubiquitin (1:200) and anti-ATF2 (1:200) antibodies (all from Abcam) at 4°C overnight, followed by incubation with a fluorophore-conjugated secondary antibody (1:200, Invitrogen). Nuclei were stained with DAPI.
Co-immunoprecipitation (Co-IP) and in vivo ubiquitination assays
Co-IP analysis was performed routinely. For in vivo ubiquitination assays, HCC cells silenced for or overexpressing ROCK2 were exposed to MG132 for 4 hours before harvesting. Specific protein complexes were immunoprecipitated from cell lysates with anti-ATF2 or anti-MKP1 antibodies. The ubiquitination levels of ATF2 and MKP1 were detected in western blot assays using an anti-ubiquitin antibody.
Statistical analysis
All data were analysed with GraphPad Prism 5 (GraphPad software, USA) and are expressed as the mean ± the standard deviation. The student t-test (two-tailed distribution) was used to analyse differences. In addition, the logistic regression model was used to analyse the invasion and metastasis data in univariate and multivariate ways. P < 0.05 was considered significant.
Results
MKP1 is significantly downregulated in HCC and correlates with the overall survival of HCC patients
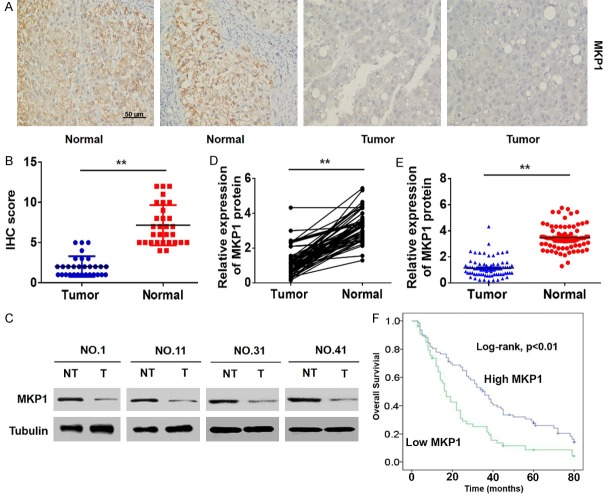
To determine the role of MKP1 in the development and progression of HCC, we initially detected its expression in 132 samples of HCC and their adjacent normal tissues by immunohistochemistry (IHC). We found that MKP1 was downregulated in HCC (Figure 1A and 1B). Western blotting assays of HCC tissues (Figure 1C-E) supported these data, confirming that the expression of MKP1 is low in HCC.
Figure 1.
MKP1 is significantly downregulated in HCC. A and B. The expression of MKP1 in HCC and adjacent normal liver tissues was analysed by IHC. C-E. Representative western blot and quantitative analysis of MKP1 expression in tissues. T: tumours, NT: non-tumours; **P < 0.01. F. Kaplan-Meier survival curve of 132 patients with HCC. *P < 0.01, logarithmic rank test.
Next, we analyzed the relationship between the expression of MKP1 and the clinicopathological features of the HCC patients. We found that low expression of MKP1 was significantly correlated with microsatellite formation and vascular infiltration in advanced tumors (Table S2). Importantly, Kaplan-Meier analysis showed that the overall survival time of patients with low MKP1 expression was significantly shorter than that of patients with high MKP1 expression (Figure 1F). In addition, univariate and multivariate logistic regression analyses showed that MKP1 was an independent predictor of microsatellite formation and vascular invasion in HCC (Table S3). These data suggest that MKP1 is expressed at low levels in HCC and is an indicator of poor prognosis.
MKP1 suppresses migration and invasion of HCC in vivo and in vitro
Because MKP1 expression was significantly associated with advanced tumour microsatellite formation and vascular invasion [17], we hypothesized that MKP1 might function in HCC cell migration and invasion. First, we investigated the relationship between MKP1 protein expression and HCC cell migration and invasion in Transwell assays. The results showed that the protein expression of MKP1 in HCC cells was reduced compared to normal hepatocytes (Figure 2A, 2B) and that overexpression of MKP1 decreased the migration and invasion ability of MHCC97H and HCCLM3 cells (Figure 2C-H). In addition, western blotting assays showed that overexpression of MKP1 led to a decrease in matrix metalloproteinase (MMP) 2 and MMP9 expression (Figure 2I, 2J).
Figure 2.
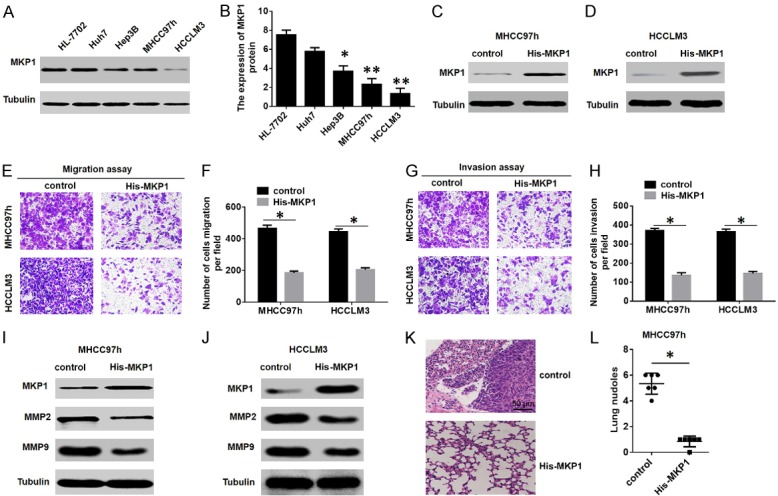
MKP1 inhibits HCC migration and invasion in vitro and in vivo. A and B. Western blot analysis of the expression of MKP1 in HCC cell lines. C and D. Western blot analysis to detect the expression of MKP1 in MHCC97H and HCCLM3 cells stably transfected with a control or a His-MKP1-overexpressing plasmid. E-H. Transwell migration assays in MHCC97H and HCCLM3 cells transfected with a control or a His-MKP1-overexpressing plasmid. *P < 0.01. I and J. Western blot analysis to detect the expression of MMP2 and MMP9 in MHCC97H and HCCLM3 cells transfected with a control or a His-MKP1-overexpressing plasmid. K and L. Incidence of lung metastasis in the MHCC97h-control and MHCC97h-His-MKP1 groups. *P < 0.01.
We further examined the effects of MKP1 on HCC metastasis by injecting normal control (NC) and Flag-MKP1 MHCC97H cells into the caudal veins of nude mice. Histologic analysis by H&E staining of serial lung sections revealed that the number of HCC lung micrometastases was significantly decreased in the Flag-MKP1 group (Figure 2K, 2L). These data demonstrate that MKP1 suppresses HCC cell metastasis ability both in vivo and in vitro.
ROCK2 regulates MKP1 protein expression to induce HCC cell migration and invasion
The recent works of several groups, including ours, have shown that ROCK2 is overexpressed in HCC and contributes to cell migration and invasion, while ROCK2 knockout inhibits the growth of HCC [16,18]. Importantly, we previously found that ROCK2 silencing resulted in an increased MKP1 protein level (Table S1). Therefore, we hypothesized that ROCK2 influences the progression of HCC by regulating the expression of MKP1. To investigate whether ROCK2 regulates MKP1 expression, we first detected the levels of ROCK2 in various HCC cells. Western blotting assays showed that ROCK2 was overexpressed in HCC cells compared to normal hepatocytes (Figure 3A). In addition, the downregulation of ROCK2 increased MKP1 expression (Figure 3B), while the overexpression of ROCK2 significantly decreased MKP1 protein expression in HCC cells (Figure 3C). These results suggest that the low expression of MKP1 is related to the overexpression of ROCK2, and MKP1 may be regulated by ROCK2.
Figure 3.
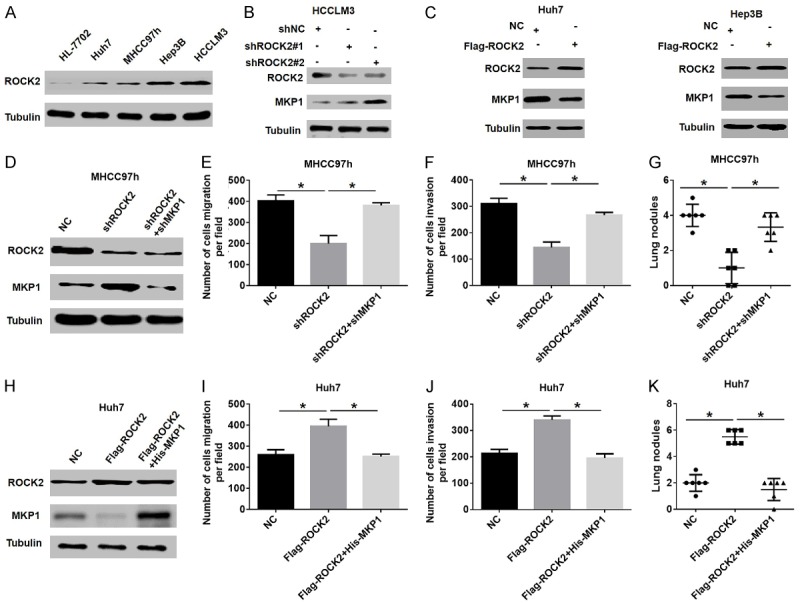
ROCK2 regulates MKP1 protein expression to influence HCC migration and invasion. A. Western blot analysis of ROCK2 expression in human normal hepatocytes and HCC cell lines. B. Western blot analysis of ROCK2 and MKP1 expression in HCCLM3 cells stably transfected with shNC or shROCK2 plasmid. C. Western blot analysis of ROCK2 and MKP1 expression in HCC cells stably transfected with a control or FLAG-ROCK2-overexpressing plasmid. D. Western blot analysis of ROCK2 and MKP1 expression in different groups of MHCC97h cells. E and F. The metastasis ability of HCC cells stably transfected with shROCK2 in the presence or absence of shMKP1 was determined by migration and invasion assays. *P < 0.05. G. The quantification of the nodules of lung metastasis in the indicated groups is shown. *P < 0.05. H. ROCK2 and MKP1 protein levels in HCC cells were detected by western blot, and stably transfected with Flag-ROCK2 in the presence or absence of His-MKP1. I and J. The metastasis ability of HCC cells stably transfected with Flag-ROCK2 in the presence or absence of His-MKP1 was determined by migration and invasion assays. *P < 0.05. K. Quantification of the nodules of lung metastasis is shown in the indicated groups. *P < 0.05.
Next, to investigate whether ROCK2 mediates the invasion and metastasis of HCC by regulating MKP1, we reduced the expression of MKP1 in ROCK2-knockdown HCC cells and then assessed cell migration and invasion. Western blot analysis showed that ROCK2 silencing increased MKP1 expression and significantly reduced the migration and invasion abilities of HCC, while the downregulation of MKP1 in ROCK2-silenced HCC cells significantly reversed this effect (Figure 3D-F). In addition, in vivo metastasis analysis showed that the reduction in MKP1 levels decreased the incidence of lung metastasis in the ROCK2-silenced-HCC group (Figure 3G). In contrast, rescue experiments indicated that enhanced MKP1 expression could be partially reversed by the pro-metastasis ability induced by overexpression of ROCK2 in HCC cells (Figure 3H-K). Therefore, these results suggest that MKP1 is crucial for ROCK2-mediated metastasis of HCC.
ROCK2 and MKP1 protein levels are inversely correlated, but their mRNA levels are positively correlated in HCC tissues
To confirm the relationship between ROCK2 and MKP1 in HCC, we first examined the expression of the two proteins in HCC tissues and analyzed their correlation. Western blot assays showed that ROCK2 was overexpressed in HCC tissue samples, while MKP1 was downregulated (Figure 4A). Consistent with the Western blot data, IHC assays confirmed these results (Figure 4B). In addition, scatter plot analysis showed that the expression of ROCK2 and MKP1 protein expression levels were negatively correlated (Figure 4C). Unexpectedly, further analysis of The Cancer Genome Atlas (TCGA) datasets showed that the mRNA level of MKP1 was decreased in HCC tissues compared to normal tissues, regardless of node metastasis status or tumor grade (Figure S1). This result prompted us to measure the mRNA levels of ROCK2 and MKP1 in HCC tissues by qRT-PCR. Our results indicated that the mRNA levels of ROCK2 and MKP1 were significantly elevated in HCC tissues compared with corresponding non-tumor tissues (Figure 4D and 4E). Moreover, the mRNA level of ROCK2 was positively correlated with MKP1 mRNA expression in HCC tissues (Figure 4F). Additionally, Kaplan-Meier analysis showed that the overall survival of patients with high MKP1 mRNA expression was significantly shorter than that of the patients with low MKP1 mRNA expression (Figure S2). Together, these data strongly suggest that overexpression of ROCK2 results in discordance between MPK1 protein and its mRNA in HCC.
Figure 4.
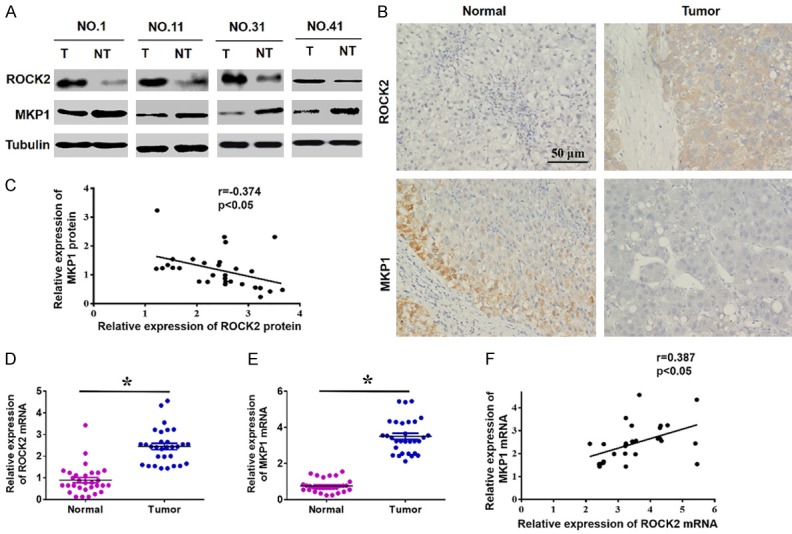
Analysed the protein and mRNA levels of ROCK2 and MKP1 in HCC tissues. A. Western blot analysis of ROCK2 and MKP1 expression in tissues (T: tumours, NT: non-tumours). B. Representative IHC staining of ROCK2 and MKP1 in HCC. C. Scatter plot analysis showing that ROCK2 and MKP1 protein expression are negatively correlated in HCC. *P < 0.05. D and E. qRT-PCR analysis of the mRNA level of ROCK2 and MKP1 in HCC specimens. *P < 0.05. F. Scatter plots showing the positive linear correlation between the mRNA expression of ROCK2 and MKP1 in HCC tissues. *P < 0.05.
ROCK2 overexpression leads to increased MKP1 mRNA expression through activation of ERK1/2-ATF2 signaling
To explore the mechanism by which ROCK2 overexpression leads to the incongruency between MPK1 protein and mRNA levels, we initially analyzed the transcription regulation mechanism of MKP1. A prior study has demonstrated that MKP1 is a target gene of ATF2 [19]. Therefore, we speculated that ROCK2 regulates MKP1 through ATF2. To test this hypothesis, we first measured the expression of MKP1 in Huh7 cells with altered ATF2 levels. We found that ATF2 knockdown significantly reduced the expression of MKP1 in HCC cells (Figure 5A, 5B), while up-regulation of ATF2 had the opposite effect (Figure 5C, 5D). These findings suggested that ATF2 regulates the expression of MKP1 in HCC cells. Next, we attempted to clarify whether ROCK2 affects the expression of MKP1 through ATF2. For this purpose, we measured the changes of ATF2 and MKP1 expression upon ROCK2 knockdown in HCC cells. Interestingly, our results showed that ROCK2 knockdown significantly reduced the expression of ATF2 and increased the protein expression of MKP1 in HCC cells, while the mRNA expression of MKP1 was decreased (Figure 5E, 5F). In contrast, the overexpression of ROCK2 in HCC cells increased the expression of ATF2 expression and decreased the protein expression of MKP1, while the MKP1 mRNA level was increased (Figure 5G, 5H).
Figure 5.
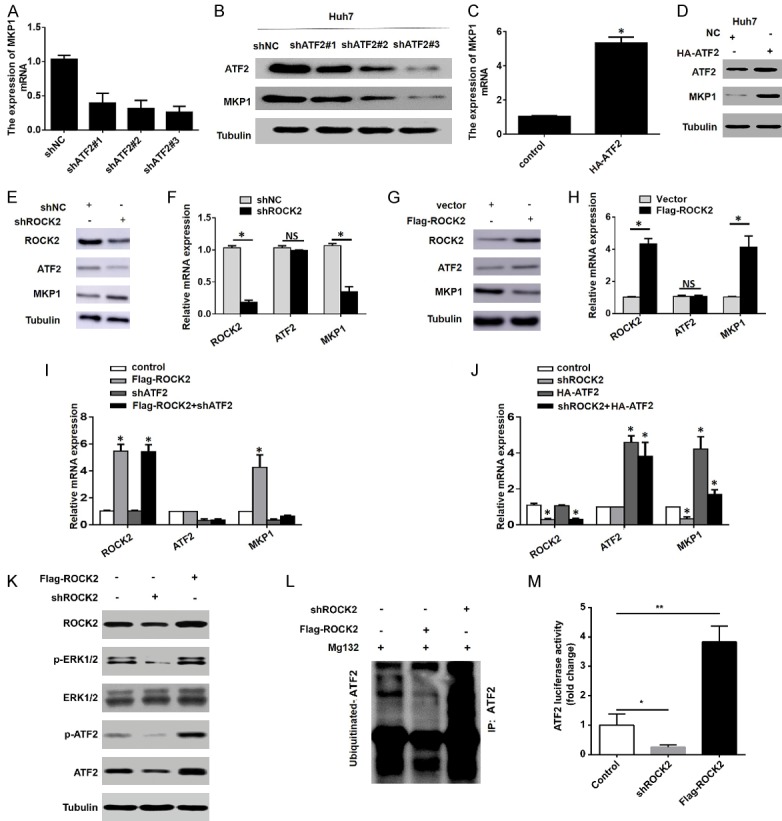
ROCK2 overexpression leads to increased MKP1 mRNA expression through activating ERK1/2-ATF2 signal. A and B. The mRNA expression of MKP1 in Huh7 cells transfected with shNC or shATF2 plasmids was detected by qRT-PCR and western blot analysis. C and D. qRT-PCR and western blot assays were used to detect the expression of MKP1 in Huh7 cells transfected with a control or HA-ATF2 overexpressing plasmid. *P < 0.05. E-H. The expression of ROCK2, ATF2 and MKP1 was detected by transfecting Huh7 cells with the shROCK2 plasmid. I and J. The mRNA expression levels of ROCK2, ATF2 and MKP1 in Huh7 cells transfected with the different plasmids. *P < 0.05. K. The expression of ROCK2, p-ERK1/2, ERK1/2, p-ATF2 (T71) and ATF2 were detected by transfecting Huh7 cells with ROCK2 upregulation or downregulation. L. The ubiquitylation levels of ATF2 was detected by western bolt. M. The transcription activity of ATF2 was measured by luciferase reporting system. *P < 0.05; **P < 0.01.
Next, we aimed to clarify whether ROCK2 affects the mRNA expression of MKP1 through ATF2. For this purpose, we overexpressed ROCK2 in HCC cells stably silenced for ATF2 expression. qRT-PCR assays showed that overexpression of ROCK2 had no effect on the mRNA expression of MKP1 in these cells (Figure 5I). In contrast, overexpression of ATF2 was associated with an increase in MKP1 mRNA in ROCK2-silenced cells (Figure 5J). These results confirmed that overexpression of ROCK2 leads to increased mRNA expression of MKP1 through ATF2.
We next examined why ROCK2 regulates the protein expression of ATF2, but not its mRNA expression (Figure 5E-H). Studies have confirmed that phosphorylation of ATF2 at Thr-71 by ERK1/2 causes increased transcriptional activity [20], and phosphorylation of ATF2 protects it from ubiquitination and degradation [21]. However, ROCK2 has been reported to mediate the activation of ERK1/2 [22]. Thus, we speculated that ROCK2 impacts the expression and activation of ATF2 through ERK1/2. To verify this speculation, we measured the expression of ERK1/2, p-ERK1/2, ATF2 and p-ATF2 in HCC cells with altered ROCK2 levels. The results showed that decreased ROCK2 expression reduced the levels of p-ERK1/2, P-ATF2 and ATF2, whereas ROCK2 overexpression increased the levels of these proteins (Figure 5K). Furthermore, we found that upregulation of ROCK2 reduced the ubiquitination levels of ATF2 and enhanced the transcriptional activity of ATF2, while downregulation of ROCK2 increased the ubiquitination levels of ATF2 and inhibited the transcriptional activity of ATF2 (Figure 5L, 5M). Taken together, these results confirm that overexpression of ROCK2 affects the expression of MKP1 by activating the ERK1/2-ATF2 signal, leading to increased MKP1 mRNA expression.
ROCK2 regulates MKP1 expression by inducing its ubiquitination in HCC cells
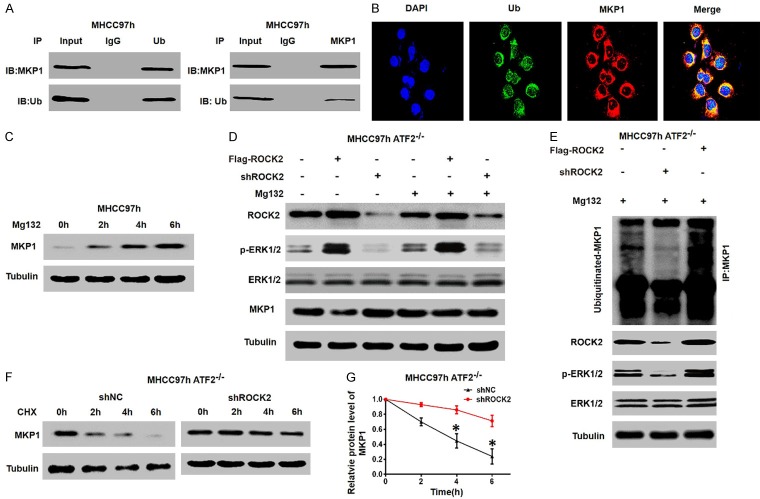
To explore the mechanisms through which ROCK2 overexpression leads to decreased in MKP1 protein levels, we first studied the ubiquitin-mediated degradation of MKP1 in HCC cells. Immunoprecipitation (IP) assays showed that MKP1 is ubiquitinated in MHCC97H cells (Figure 6A), and confocal microscopy further confirmed this result (Figure 6B). In addition, treatment of HCC cells with the proteasome inhibitor MG132 resulted in significant accumulation of endogenous MKP1 (Figure 6C). These results suggest that MKP1 is degraded by UPS in HCC cells.
Figure 6.
ROCK2 induces the ubiquitination of MKP1 and inhibits MKP1 protein einducxpression by activating ERK1/2. A. Co-IP was performed to investigate the interaction between endogenous MKP1 and ubiquitin (Ub) in MHCC97h cells. B. MHCC97h cells were co-stained with DAPI indicates the nucleI (blue); an anti-UB antibody (green); and an anti-MKP1 antibody (red). C. MHCC97h cells were treated with MG132 (15 µmol/l) and MKP1 levels were measured. D. MHCC97h cells transduced with Flag-ROCK2 and shROCK2 plasmid were treated with MG132 (15 µmol/l), and western blot was performed to measure the expression levels of the indicated proteins. E. MHCC97h-ATF2-/- cells transduced with Flag-ROCK2 and shROCK2 plasmid and treated with MG132 (15 µmol/l). Western blot was performed to measure the ubiquitylation levels of MKP1 and the expression levels of the indicated proteins. F and G. Representative western blot showing that ROCK2 silencing inhibits the degradation of MKP1 in HCC cells. HCC cells were treated with CHX (100 mg/ml) and western blot analysis was performed at the designated time points. *P < 0.05.
A study previously demonstrated that activated ERK1/2 can trigger MKP-1 degradation via the ubiquitin-proteasome pathway [23], which led us to determine whether the ROCK2-ERK1/2 signal is involved in regulation of MKP1 degradation. We initially silenced or overexpressed ROCK2 in MHCC97h-ATF2-/- cells in which ATF2 had been knockout by the CRISPR/Cas9 system and analyzed MKP1 expression. We found that the downregulation or overexpression of ROCK2 had no significant effect on the expression of MKP1 in MHCC97h-ATF2-/- cells treated with MG132 (Figure 6D). Furthermore, ROCK2 knockdown significantly reduced ubiquitination of MKP1, while ectopic expression of ROCK2 increased the ubiquitination of MKP1 in MHCC97h-ATF2-/- cells (Figure 6E). In addition, an analysis of degradation kinetics showed that decreased the ROCK2 expression significantly prolonged the half-life of MKP1 in MHCC97h-ATF2-/- cells (Figure 6F, 6G). Thus, these findings demonstrate that ROCK2 activates the ERK1/2 signal, which enhances ubiquitination and degradation of the MKP1 protein.
Relationship among ROCK2, ATF2 and MKP1 in HCC tissues
Finally, to determine whether the relationship among ROCK2, ATF2 and MKP1 is clinically relevant, we measured the expression of ROCK2 protein, ATF2 protein, and MKP1 mRNA and protein in HCC tissues. The scatter plot analysis indicated that ROCK2 and ATF2 protein expression were positively correlated (Figure 7A); ROCK2 protein expression and MKP1 mRNA expression were positively correlated (Figure 7B); ATF2 protein expression and MKP1 mRNA expression were positively correlated (Figure 7C); and the expression of MKP1 protein and mRNA were negatively correlated in HCC tissues (Figure 7D). In summary, these results verify that overexpression of ROCK2 leads to different effects on the expression of MKP1 at the protein and mRNA levels; ROCK2 activates the ERK1/2-ATF2 signaling, which leads to an increase in MKP1 mRNA expression. At the same time, ROCK2 activates ERK1/2 to promote the ubiquitin-mediated degradation of MKP1 and therefore metastasis of HCC (Figure 7E).
Figure 7.
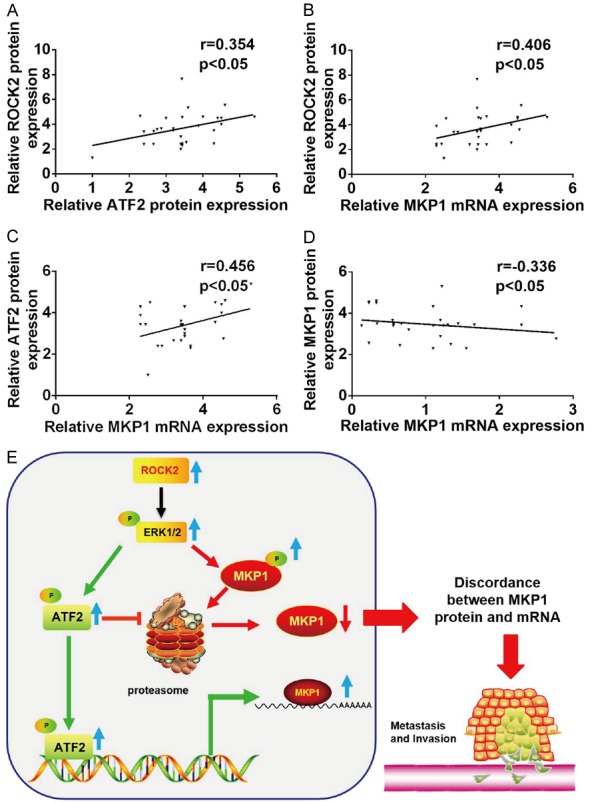
The relation of expression among ROCK2, ATF2 and MKP1. A. Scatter plots showing the correlation between ROCK2 protein and ATF2 protein expression. B. Scatter plots showing the correlation between ROCK2 protein and MKP1 mRNA expression. C. Scatter plots showing the correlation between ATF2 protein and MKP1 mRNA expression. D. Scatter plots showing the correlation between MKP1 protein and MKP1 mRNA expression. E. Model summarizing the mechanism that ROCK2 disturbs the protein and mRNA level of MKP1 in human HCC progression.
Discussion
HCC is one of the most common malignant tumours worldwide and has the highest mortality [24]. Although some progress has been made in HCC clinical detection and treatment of HCC in recent years, the metastasis and recurrence rates after radical resection are still high [25]. Here, we found a new tumor suppressor gene, MKP1, which is associated with the invasion and metastasis of HCC. MKP1 is a threonine-tyrosine bispecific phosphatase [26]. Studies conducted to investigate the expression and function of MKP1 in tumous have reached contradictory results. Knockout of MKP1 in pancreatic cancer cells reduces the incidence of cancer [4,27]. A different study, however, showed that MKP1 expression is low in HCC, which indicates that MKP1 plays a role as a tumour suppressor [28]. In this study, we found that MKP1 expression is low in HCC tissues and cells, and MKP1 acts as a tumour suppressor. Our results indicate that the overall survival of patients with low MKP1 expression is significantly decreased. In addition, univariate and multivariate analyses showed that low MKP1 protein expression is an independent predictor of poor prognosis in HCC patients. Furthermore, we have provided in vivo and in vitro evidence that MKP1 can inhibit HCC cells metastasis. Moreover, our data suggest that the mechanism of through which MKP1 inhibits metastasis formation may be related to the decreased expression of MMP2 and MMP9.
We also explored the causes of low expression of MKP1 in HCC and identified a new mechanism that regulates this expression. Our data suggest that low MKP1 expression in HCC is closely related to ROCK2 overexpression. ROCK2 is upregulated in many malignant tumors, including HCC, and the overexpression of ROCK2 is related to a poor prognosis [29]. Additionally, studies from several groups, including ours, have shown that increased ROCK2 expression promotes metastasis of HCC cells. Here, we report that downregulation of ROCK2 increases the expression of MKP1, and this effect is counteracted by the downregulation of MKP1 in ROCK2-silenced cells. We also found that knockdown of ROCK2 significantly reduces the migration and invasion abilities of HCCLM3 cells in a partly MKP1-dependent manner. In addition, our results showed that ROCK2 induces the ubiquitination and degradation of MKP1 in HCC cells.
Studies have shown that DUSP1 dephosphorylates and inactivates ERK, blocking the activation of ERK signaling [30,31]. However, sustained ERK1/2 activation has been shown to be correlated with proteolysis of MKP1, and forced expression of constitutively active MKK1/2 in several cells types has resulted in MKP1 ubiquitination and proteolysis, thus facilitating sustained kinase activation for long periods [23]. Consistently, we also found that ERK1/2 signaling activated by ROCK2 can trigger degradation of MKP-1 via the ubiquitin-proteasome pathway. One possible explanation is that activated ERK1/2 increases MKP1 expression and phosphorylates the extreme MKP1 C-terminus, thereby elevating phosphatase activity and stabilizing MKP1, which leads to de-phosphorylation of ERK1/2 and net transient activation of ERK signaling. However, an increased in the duration or strength of ERK activation, may phosphorylate MKP1 at sites other than the two extreme C-terminal Ser residues and facilitate subsequent ubiquitination and degradation. By triggering MKP1 downregulation, ERK achieves sustained activation [23].
Recently, studies on the relationship between the transcriptome and the proteome have highlighted that mRNA and protein expression is not consistent in some genes, and it has been speculated that post-translational modifications (PTMs) might be involved in this phenomenon [32,33]. Recently, Yan et al. showed that FAT10 overexpression promotes WISP1 mRNA expression by stabilizing β-catenin and directly degrades the WISP1 protein in HCC [34]. Here, we demonstrated that ROCK2, which plays an important role in PTM, is involved in the inconsistent expression of MKP1 protein and mRNA. Our results showed that the overexpression of ROCK2 resulted in a decrease in MKP1 protein and an increase in MKP1 mRNA in HCC cells, while knockout of ROCK2 resulted in an increase in MKP1 protein and a decrease in MKP1 mRNA. Our data showed that ATF2 activates the expression of MKP1 mRNA in HCC cells. Notably, our results revealed that the overexpression of ROCK2 increases the expression of MKP1 mRNA by activating the ERK1/2-ATF2 pathway. At the same time, ROCK2 activates ERK1/2 to downregulate the expression of MKP1 protein and promote metastasis of HCC by promoting the ubiquitination and degradation of MKP1. Although the underlying molecular mechanism remains unclear and further studies in this regard are required, the regulation of MKP1 by ROCK2 may provide new insights into the mechanism of tumorigenesis in several types of cancer including HCC.
In summary, to our knowledge, our study is the first to demonstrate that ROCK2 disturbs the expression of the MKP1 protein to promote metastasis and invasion of HCC. Thus, our data provide new evidence for the biological and clinical significance of MKP1 as a potential biomarker.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81760523, 81560117 and 81660409), Project of Jiangxi Provincial Department of Science and Technology (20171BAB215024), Jiangxi Provincial Department of Health (2017A264) and Jiangxi Provincial Department of Education (GJJ160251).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Sayiner M, Golabi P, Younossi ZM. Disease burden of hepatocellular carcinoma: a global perspective. Dig Dis Sci. 2019;64:910–917. doi: 10.1007/s10620-019-05537-2. [DOI] [PubMed] [Google Scholar]
- 2.Kumari R, Sahu MK, Tripathy A, Uthansingh K, Behera M. Hepatocellular carcinoma treatment: hurdles, advances and prospects. Hepat Oncol. 2018;5:HEP08. doi: 10.2217/hep-2018-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Low HB, Zhang Y. Regulatory roles of MAPK phosphatases in cancer. Immune Netw. 2016;16:85–98. doi: 10.4110/in.2016.16.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen J, Zhang Y, Yu H, Shen B, Liang Y, Jin R, Liu X, Shi L, Cai X. Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med. 2016;5:2061–2068. doi: 10.1002/cam4.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moncho-Amor V, Ibañez de Cáceres I, Bandres E, Martínez-Poveda B, Orgaz JL, Sánchez-Pérez I, Zazo S, Rovira A, Albanell J, Jiménez B, Rojo F, Belda-Iniesta C, García-Foncillas J, Perona R. DUSP1/MKP1 promotes angiogenesis, invasion and metastasis in non-small-cell lung cancer. Oncogene. 2011;30:668–678. doi: 10.1038/onc.2010.449. [DOI] [PubMed] [Google Scholar]
- 6.Christmann M, Tomicic MT, Aasland D, Kaina B. A role for UV-light-induced c-Fos: stimulation of nucleotide excision repair and protection against sustained JNK activation and apoptosis. Carcinogenesis. 2007;28:183–190. doi: 10.1093/carcin/bgl119. [DOI] [PubMed] [Google Scholar]
- 7.Cao T, Yang D, Zhang X, Wang Y, Qiao Z, Gao L, Liang Y, Yu B, Zhang P. FAM3D inhibits glucagon secretion via MKP1-dependent suppression of ERK1/2 signaling. Cell Biol Toxicol. 2017;33:457–466. doi: 10.1007/s10565-017-9387-8. [DOI] [PubMed] [Google Scholar]
- 8.Shen J, Zhou S, Shi L, Liu X, Lin H, Yu H, Xiaoliang , Tang J, Yu T, Cai X. DUSP1 inhibits cell proliferation, metastasis and invasion and angiogenesis in gallbladder cancer. Oncotarget. 2017;8:12133–12144. doi: 10.18632/oncotarget.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candas D, Lu CL, Fan M, Chuang FY, Sweeney C, Borowsky AD, Li JJ. Mitochondrial MKP1 is a target for therapy-resistant HER2-positive breast cancer cells. Cancer Res. 2014;74:7498–7509. doi: 10.1158/0008-5472.CAN-14-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases. 2014;5:e29846. doi: 10.4161/sgtp.29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schofield AV, Bernard O. Rho-associated coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem Mol Biol. 2013;48:301–316. doi: 10.3109/10409238.2013.786671. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Ke J, Wang Q, Qian H, Yang L, Zhang X, Xiao J, Ding H, Shan X, Liu Q, Xiao Y, Bao B, Huang H. Upregulation of ROCK2 in gastric cancer cell promotes tumor cell proliferation, metastasis and invasion. Clin Exp Med. 2017;17:519–529. doi: 10.1007/s10238-016-0444-z. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Zhou W, Yuan R, Chen L, Liu T, Huang D, Hao L, Xie Y, Shao J. ROCK2 promotes HCC proliferation by CEBPD inhibition through phospho-GSK3beta/beta-catenin signaling. FEBS Lett. 2015;589:1018–1025. doi: 10.1016/j.febslet.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Wong CC, Wong CM, Tung EK, Man K, Ng IO. Rho-kinase 2 is frequently overexpressed in hepatocellular carcinoma and involved in tumor invasion. Hepatology. 2009;49:1583–1594. doi: 10.1002/hep.22836. [DOI] [PubMed] [Google Scholar]
- 15.Ai N, Li B, Li L, Li Z, Ji H, Yang G, Yin F. MicroRNA-466 inhibits cancer cell migration and invasion in hepatocellular carcinoma by indirectly mediating the downregulation of ROCK2. Exp Ther Med. 2019;18:1493–1499. doi: 10.3892/etm.2019.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang D, Du X, Yuan R, Chen L, Liu T, Wen C, Huang M, Li M, Hao L, Shao J. Rock2 promotes the invasion and metastasis of hepatocellular carcinoma by modifying MMP2 ubiquitination and degradation. Biochem Biophys Res Commun. 2014;453:49–56. doi: 10.1016/j.bbrc.2014.09.061. [DOI] [PubMed] [Google Scholar]
- 17.Vu HN, Miller WJ, O’Connor SA, He M, Schafer PH, Payvandi F, Muller GW, Stirling DI, Libutti SK. CC-5079: a small molecule with MKP1, antiangiogenic, and antitumor activity. J Surg Res. 2010;164:116–125. doi: 10.1016/j.jss.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Niu Y, Tang G. miR-185-5p targets ROCK2 and inhibits cell migration and invasion of hepatocellular carcinoma. Oncol Lett. 2019;17:5087–5093. doi: 10.3892/ol.2019.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breitwieser W, Lyons S, Flenniken AM, Ashton G, Bruder G, Willington M, Lacaud G, Kouskoff V, Jones N. Feedback regulation of p38 activity via ATF2 is essential for survival of embryonic liver cells. Genes Dev. 2007;21:2069–2082. doi: 10.1101/gad.430207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouwens DM, de Ruiter ND, van der Zon GC, Carter AP, Schouten J, van der Burgt C, Kooistra K, Bos JL, Maassen JA, van Dam H. Growth factors can activate ATF2 via a two-step mechanism: phosphorylation of Thr71 through the Ras-MEK-ERK pathway and of Thr69 through RalGDS-Src-p38. EMBO J. 2002;21:3782–3793. doi: 10.1093/emboj/cdf361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs SY, Tappin I, Ronai Z. Ronai, stability of the ATF2 transcription factor is regulated by phosphorylation and dephosphorylation. J Biol Chem. 2000;275:12560–12564. doi: 10.1074/jbc.275.17.12560. [DOI] [PubMed] [Google Scholar]
- 22.Matsubara M, Bissell MJ. Inhibitors of Rho kinase (ROCK) signaling revert the malignant phenotype of breast cancer cells in 3D context. Oncotarget. 2016;7:31602–31622. doi: 10.18632/oncotarget.9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YW, Chuang SM, Yang JL. ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J Biol Chem. 2003;278:21534–21541. doi: 10.1074/jbc.M301854200. [DOI] [PubMed] [Google Scholar]
- 24.Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY) 2018;43:13–25. doi: 10.1007/s00261-017-1209-1. [DOI] [PubMed] [Google Scholar]
- 25.Jindal A, Thadi A, Shailubhai K. Hepatocellular carcinoma: etiology and current and future drugs. J Clin Exp Hepatol. 2019;9:221–232. doi: 10.1016/j.jceh.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases--regulating the immune response. Nat Rev Immunol. 2007;7:202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 27.Checker R, Gambhir L, Sharma D, Kumar M, Sandur SK. Plumbagin induces apoptosis in lymphoma cells via oxidative stress mediated glutathionylation and inhibition of mitogen-activated protein kinase phosphatases (MKP1/2) Cancer Lett. 2015;357:265–278. doi: 10.1016/j.canlet.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 28.Tsujita E, Taketomi A, Gion T, Kuroda Y, Endo K, Watanabe A, Nakashima H, Aishima S, Kohnoe S, Maehara Y. Suppressed MKP-1 is an independent predictor of outcome in patients with hepatocellular carcinoma. Oncology. 2005;69:342–347. doi: 10.1159/000089766. [DOI] [PubMed] [Google Scholar]
- 29.Wong CC, Wong CM, Tung EK, Au SL, Lee JM, Poon RT, Man K, Ng IO. The microRNA miR-139 suppresses metastasis and progression of hepatocellular carcinoma by down-regulating Rho-kinase 2. Gastroenterology. 2011;140:322–331. doi: 10.1053/j.gastro.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Shi Y, Du Y, Ning X, Liu N, Huang D, Liang J, Xue Y, Fan D. Dual-specificity phosphatase DUSP1 protects overactivation of hypoxia-inducible factor 1 through inactivating ERK MAPK. Exp Cell Res. 2005;309:410–418. doi: 10.1016/j.yexcr.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Sun L, Han J, Zheng W, Peng W. DUSP1/MKP-1 regulates proliferation and apoptosis in keratinocytes through the ERK/Elk-1/Egr-1 signaling pathway. Life Sci. 2019;223:47–53. doi: 10.1016/j.lfs.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell. 2016;165:535–550. doi: 10.1016/j.cell.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Mertins P, Mani DR, Ruggles KV, Gillette MA, Clauser KR, Wang P, Wang X, Qiao JW, Cao S, Petralia F, Kawaler E, Mundt F, Krug K, Tu Z, Lei JT, Gatza ML, Wilkerson M, Perou CM, Yellapantula V, Huang KL, Lin C, McLellan MD, Yan P, Davies SR, Townsend RR, Skates SJ, Wang J, Zhang B, Kinsinger CR, Mesri M, Rodriguez H, Ding L, Paulovich AG, Fenyö D, Ellis MJ, Carr SA NCI CPTAC. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature. 2016;534:55–62. doi: 10.1038/nature18003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan J, Lei J, Chen L, Deng H, Dong D, Jin T, Liu X, Yuan R, Qiu Y, Ge J, Peng X, Shao J. Human leukocyte antigen F locus adjacent transcript 10 overexpression disturbs WISP1 protein and mRNA expression to promote hepatocellular carcinoma progression. Hepatology. 2018;68:2268–2284. doi: 10.1002/hep.30105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.