Abstract
Background
Although highly effective in the treatment of obstructive sleep apnoea (OSA), continuous positive airway pressure (CPAP) is not universally accepted by users. Educational, supportive and behavioural interventions may help people with OSA initiate and maintain regular and continued use of CPAP.
Objectives
To assess the effectiveness of educational, supportive, behavioural, or mixed (combination of two or more intervention types) strategies that aim to encourage adults who have been prescribed CPAP to use their devices.
Search methods
Searches were conducted on the Cochrane Airways Group Specialised Register of trials. Searches are current to 29 April 2019.
Selection criteria
We included randomised controlled trials (RCTs) that assessed intervention(s) designed to inform participants about CPAP/OSA, to support them in using CPAP, or to modify their behaviour to increase use of CPAP devices.
Data collection and analysis
We assessed studies to determine their suitability for inclusion in the review. Data were extracted independently and were entered into RevMan for analysis. 'Risk of bias' assessments were performed, using the updated 'Risk of bias 2' tool, for the primary outcome, CPAP usage. Study‐level 'Risk of bias' assessments were performed using the original 'Risk of bias' tool. GRADE assessment was performed using GRADEpro.
Main results
Forty‐one studies (9005 participants) are included in this review; 16 of these studies are newly identified with updated searches. Baseline Epworth Sleepiness Scale (ESS) scores indicate that most participants suffered from excessive daytime sleepiness. The majority of recruited participants had not used CPAP previously. When examining risk of bias for the primary outcome of hourly machine usage/night, 58.3% studies have high overall risk (24/41 studies), 39.0% have some concerns (16/41 studies), and 2.4% have low overall risk (1/41 studies).
We are uncertain whether educational interventions improve device usage, as the certainty of evidence was assessed as very low. We were unable to perform meta‐analyses for number of withdrawals and symptom scores due to high study heterogeneity.
Supportive interventions probably increase device usage by 0.70 hours/night (95% confidence interval (CI) 0.36 to 1.05, N = 1426, 13 studies, moderate‐certainty evidence), and low‐certainty evidence indicates that the number of participants who used their devices ≥ 4 hours/night may increase from 601 to 717 per 1000 (odds ratio (OR), 1.68, 95% CI 1.08 to 2.60, N = 376, 2 studies). However, the number of withdrawals may also increase from 136 to 167 per 1000 (OR 1.27, 95% CI 0.97 to 1.66, N = 1702, 11 studies, low‐certainty evidence). Participants may experience small improvements in symptoms (ESS score ‐0.32 points, 95% CI ‐1.19 to 0.56, N = 470, 5 studies, low‐certainty evidence), and we are uncertain whether quality of life improves with supportive interventions, as the certainty of evidence was assessed as very low.
When compared with usual care, behavioural interventions produce a clinically‐meaningful increase in device usage by 1.31 hours/night (95% CI 0.95 to 1.66, N = 578, 8 studies, high‐certainty evidence), probably increase the number of participants who used their machines ≥ 4 hours/night from 371 to 501 per 1000 (OR 1.70, 95% CI 1.20 to 2.41, N = 549, 6 studies, high‐certainty evidence), and reduce the number of study withdrawals from 146 to 101 per 1000 (OR 0.66, 95% CI 0.44 to 0.98, N = 939, 10 studies, high‐certainty evidence). Behavioural interventions may reduce symptoms (ESS score ‐2.42 points, 95% CI ‐4.27 to ‐0.57, N = 272, 5 studies, low‐certainty evidence), but probably have no effect on quality of life (Functional Outcomes of Sleep Questionnaire (FOSQ), standardised mean difference (SMD) 0.00, 0.95% CI ‐0.26 to 0.26, N = 228, 3 studies, moderate‐certainty evidence). We are uncertain whether behavioural interventions improve apnoea hypopnoea index (AHI), as the certainty of evidence was assessed as very low.
We are uncertain if mixed interventions improve device usage, increase the number of participants using their machines ≥ 4 hours/night, reduce study withdrawals, improve quality of life, or reduce anxiety symptoms, as the certainty of evidence for these outcomes was assessed to be very low. Symptom scores via the ESS could not be measured due to considerable heterogeneity between studies.
Authors' conclusions
In CPAP‐naïve people with OSA, high‐certainty evidence indicates that behavioural interventions yield a clinically‐significant increase in hourly device usage when compared with usual care. Moderate certainty evidence shows that supportive interventions increase usage modestly. Very low‐certainty evidence shows that educational and mixed interventions may modestly increase CPAP usage. The impact of improved CPAP usage on daytime sleepiness, quality of life, and mood and anxiety scores remains unclear since these outcomes were not assessed in the majority of included studies. Studies addressing the choice of interventions that best match individual patient needs and therefore result in the most successful and cost‐effective therapy are needed.
Plain language summary
Do supportive, educational and behavioural interventions improve usage of continuous positive airway pressure in adults with obstructive sleep apnoea?
What is obstructive sleep apnoea (OSA) and continuous positive airway pressure (CPAP)?
Obstructive sleep apnoea (OSA) is a condition that causes interrupted breathing during sleep. People with OSA spend more time in light sleep and less time in deep sleep and consequently experience daytime sleepiness, which may affect their daily life.
Continuous positive airway pressure (CPAP) is a treatment that delivers pressurised air to keep the airway open. CPAP treatment involves a machine with three main parts: a device that fits over nose and mouth, a tube that connects the mask to the device's motor; and a motor that blows air into the tube.
Review question
We already know that CPAP treats OSA effectively in most people by improving symptoms resulting from OSA. However many people do not use their CPAP machine as much as is recommended. We wanted to look at interventions designed to educate and motivate people with OSA to use their CPAP machines more.
Study characteristics
We looked at evidence from randomised, parallel‐group, controlled studies. Following a comprehensive literature search and assessment of trials, we included 41 studies (number of participants = 9005). Most people experienced excessive daytime sleepiness and had newly diagnosed OSA. Duration of studies ranged from 28 days to two years.
Results
We grouped the trials into those that gave people a) education, b) a supportive intervention, c) behavioural intervention, and d) a mixed intervention (using all thee techniques).
We found that all types of interventions increase CPAP usage with varying levels of certainty. Behavioural therapy increases machine usage by 79 minutes per night, and ongoing supportive interventions probably increase machine use by about 42 minutes per night. Educational and mixed interventions may potentially improve machine usage, however the certainty of this evidence is very low.
We also wanted to look at other outcomes such as daytime sleepiness using the Epworth Sleepiness Scale (ESS), quality of life, depression, and apnoea hypopnoea index (measurement of pauses in breathing and slow or shallow breathing). Not all included studies consistently examined these other outcomes, however behavioural interventions may reduce daytime sleepiness.
Studies generally recruited people who were new to CPAP.
Quality of the evidence
The quality of evidence for improved CPAP adherence varied considerably across studies and study types. We were confident that the behavioural interventions improve adherence for around 70 minutes per night. The quality of evidence for educational, supportive, and mixed interventions was not as strong. The quality of evidence for OSA‐related symptoms including daytime sleepiness, quality of life, anxiety or depression was affected by the low number of studies that measured these outcomes.
Summary of findings
Background
Description of the condition
Obstructive sleep apnoea (OSA) is a common sleep‐related breathing disorder characterised by transient interruption of ventilation caused by complete or partial occlusion of the upper airway. Prolonged airway occlusion may lead to oxygen desaturation, which reduces vascular elasticity, increases coagulability and blood pressure, and predisposes to atherosclerosis (Gagnon 2014). The hypoxia and subsequent reoxygenation caused by OSA can increase blood brain barrier permeability, resulting in neurotoxicity and both medical and neuropsychiatric consequences (Bucks 2013; Canessa 2011; Devita 2017; Lochhead 2010; Olaithe 2013; Osorio 2015; Pan 2014; Stranks 2016; Verstraeten 2004; Yaffe 2011). Additionally, recurrent hypoxia and increased inspiratory effort lead to sleep fragmentation and frequent arousals from sleep.
For many individuals, these physiological changes and sleep fragmentation collectively lead to a range of symptoms: excessive daytime sleepiness (Schwab 2013), mood alterations (Garbarino 2018; Jackson 2018), impairment of cognition and memory (Delhikar 2019; Gagnon 2019; Olaithe 2013; Olaithe 2018), and changes in driving competence (Gagnon 2014; Karimi 2015; Phillipson 1993; Schwab 2013; Tregear 2009). Furthermore, OSA is associated with cardiovascular, cerebrovascular and metabolic co‐morbidities (Dong 2018; Gami 2005; Hashmi 2014; Harsch 2004; Marin 2005; Mokhlesi 2016; Peppard 2000; Punjabi 2009; Schwab 2013; Senaratna 2016; Young 2002; Young 2002a; ; ;), as well as increased mortality (Gami 2005; Marin 2005; Marshall 2008; Marshall 2014; Punjabi 2009; Yaggi 2005; Young 2008).
Phenotyping of OSA severity (mild, moderate and severe) is commonly designated by apnoea hypopnoea index (AHI) (> 5 and ≤15, 15 to 30, > 30). However, OSA is a heterogeneous disorder with different risk factors, clinical presentations, pathophysiology and morbidity (Sutherland 2018). AHI is not the only parameter characterising OSA severity. Recently developed measures of hypoxic burden may better predict cardiovascular mortality associated with OSA (Azarbarzin 2019). Patients with excessive daytime sleepiness are not only at a higher risk of cardiovascular complications, but also have significantly diminished quality of life (Mazzotti 2019). Finally, degree of daytime symptom is not tightly correlated with AHI, so patients with AHI in the mild range may experience significant impairment and patients with high AHI may be relatively asymptomatic (Garbarino 2018b; Zinchuk 2017).
Description of the intervention
The usual first line treatment for moderate to severe OSA is continuous positive airway pressure (CPAP) (Schwab 2013; Kennedy 2019), which involves the use of an airflow generator to provide a constant stream of pressurised air to splint open and maintain patency of the upper airway during the inspiratory and expiratory phases of breathing. When used throughout the entirety of sleep, CPAP eliminates nearly 100% of obstructive apneas/hypopnoeas for the majority of treated patients (Reeves‐Hoche 1994; Sawyer 2011; Sullivan 1981).
Consistent application of CPAP therapy improves the quality of sleep, normalises sleep architecture (Canessa 2011; Baker 2016), reduces daytime sleepiness, enhances neurobehavioural performance (Ancoli‐Israel 2008; Bucks 2013; Canessa 2011; Dalmases 2014; Dalmases 2015; Olaithe 2013; Zimmerman 2006); and reduces the risk of motor vehicle crashes (Findley 2000; Giles 2006; Gurubhagavatula 2017t; Hack 2000; Karimi 2015; Tregear 2009). Longitudinal studies have indicated that CPAP treatment has a protective effect on cardiovascular outcomes; patients who are compliant with CPAP achieve better blood pressure control (Haentjens 2007; Marin 2012; Martinez‐Garcia 2012; Pedrosa 2013; Pepperell 2002; Thunstrom 2016), and have reduced the risk of cardiovascular events (Campos‐Rodriguez 2014; Marin 2005; Martinez‐Garcia 2012; Myhill 2012; Wang 2017). Furthermore, adequate adherence to CPAP may slow cognitive decline (Richards 2019), and have a role in the prevention and treatment of acute stroke (Bravata 2011; Faheem 2018; Martinez‐Garcia 2009). However, it should be noted that this evidence has yet to be corroborated through randomised controlled trials (RCTs). The largest and most recent systematic review and meta‐analysis (Yu 2017) of the effect of CPAP on cardiovascular outcomes found no significant association. Notably CPAP usage was < 4 hours/night in the majority of (and all large) included RCTs. Similar nonsignficant findings were found by the SAVE trial (McEvoy 2016); both of these studies, as well as their proposed implications, are discussed at length in Appendix 1.
Despite the widespread recommendation of CPAP in the management of OSA (Fleetham 2011; Giles 2006; Patil 2019; Schwab 2013), concerns have arisen about its initial and continued acceptance among people who have to use it on a long‐term basis (i.e. the majority of patients diagnosed with OSA ). Reported adherence to CPAP ranges from 17% to 85% (Crawford 2014; Finkel 2009; Lewis 2004; Libman 2017; Lindberg 2006; Pépin 1999; Rotenberg 2016; Somers 2008; Weaver 2010; Young 2009). Eight per cent to 15% of patients refuse to accept the treatment after a single night's use, and some case series report an abandonment rate of up to 50% within one year (Bollig 2010; Krieger 1992). Frequently cited side effects leading to CPAP refusal include general discomfort, nasal congestion, abdominal bloating, mask leaks, claustrophobia, and inconvenience of regular usage (Pepin 1995). Poor adherence may also be related to the fact that CPAP requires a substantial and long‐term behavioural change. The difficulty of accomplishing such change may be further compounded by the high rates of comorbid depressive and anxiety symptoms found among patients with OSA (Chirinos 2017; Jackson 2018; Ohayon 2003; Saunamaki 2007). Moreover, CPAP therapy is not reimbursed in many countries (particularly for those with less severe OSA symptoms), further discouraging initiation of treatment, despite proven effectiveness.
Previously, CPAP use was measured through subjective self‐report or observations made within a clinical setting, each presenting its own bias. Self‐reported adherence is often overestimated and inaccurate, and how a patient behaves in a clinical setting is not generalisable to real world behaviour (Kribbs 1993). Technological advances have dramatically improved the accuracy of, and removed bias from adherence measurement through internalised digital counters or electronic microchip, now standard within CPAP devices. Despite this, the number of hours per night and the frequency of usage required to achieve and maintain therapeutic effectiveness are not well established.
Thresholds for "effective" CPAP usage may depend on the desired health benefit, which vary significantly between patients and relative to baseline severity of OSA (Bollig 2010; Campos‐Rodriguez 2005; Sawyer 2011;Stradling 2000; Wang 2017; Weaver 2007; Zimmerman 2006; ; ). Six to eight hours each night is a common clinical prescription for CPAP, but many studies use a threshold of four hours/night to define 'adherence’ (Lewis 2004; Richards 2007). This threshold likely emerged from early seminal studies (Reeves‐Hoche 1994; Kribbs 1993; Engleman 1994), in which average use was reported in the range of four hours/night, although the authors did not suggest this represented adequate or effective use. Unfortunately, not only has this threshold been widely employed in clinical trials, but it has directly impacted clinical practice in ways likely unintended by original or subsequent investigators. For example, several commonly‐used CPAP devices automatically display a happy face (or other positive feedback) on the user interface once they have reached a use threshold of four hours in a 24‐hour cycle. Additionally, some 'payors' will only cover the costs associated with CPAP for patients who use their device at or above an arbitrary (Schwab 2013) minimum of four hours per night on 70% of nights during an initiation period (e.g. Centers for Medicare and Medicaid Services; Mehrtash 2019). These practices, meant to encourage use, may have serious consequences for those aiming to address longstanding symptoms, risk factors or conditions.
Evidence suggests effectiveness of CPAP follows a dose‐response curve with benefit accruing at different thresholds for different outcomes. For example, Wang 2017 found that CPAP use < 4 hours/night improved daytime sleepiness, four to six hours/night improved all measured symptoms (sleep quality, daytime sleepiness, fatigue and depressive symptoms) while use of ≥ 6 hours produced significantly still greater improvements in all measured domains. Average nightly AHI is reduced by any CPAP use, but whether it reduces AHI to 'subthreshold' severity (AHI < 5) will depend upon baseline AHI, duration of CPAP use, and duration of sleep without CPAP (i.e. unrecorded time). However, increased use surpassing four hours/night has been associated with improvement in sleep architecture and arousal outcomes, reductions in blood pressure (Yang 2015), and reductions in the risk of cardiovascular and cerebrovascular events, while improved cognition and memory and decreased mortality (from the limited data available) likely require greater than six hours/night (Campos‐Rodriguez 2005; Zimmerman 2006). As such, maximising total CPAP use (i.e. to the full amount of recommended nightly sleep for adults, ˜8 hours/night) is optimal and preferred.
How the intervention might work
A substantial amount of studies have investigated predictors of CPAP adherence related to patient characteristics, disease characteristics, and CPAP technology. Research has demonstrated that perceived self‐efficacy, confidence, and motivation are both targetable and modifiable (Bandura 1982; Bandura 1986; Bandura 2004; Miller 1994), and correlate well with sustained and successful treatment (Mehrtash 2019). Moreover, social factors, including marital status, partner involvement and attitudes towards treatment, and partner's sleep quality, have been shown to positively influence CPAP adherence (Mehrtash 2019; Lewis 2004). Lastly, early adoption of CPAP treatment (i.e. within the first week to month of CPAP prescription) has been shown to predict long‐term adherence behaviour (Aloia 2007; Chervin 1997).
From these predictors, various interventions to improve initial acceptance and subsequent CPAP adherence have been proposed, each varying in duration, intensity, frequency and modality. Some approaches emphasise that increasing knowledge of OSA, CPAP and associated health outcomes may directly promote CPAP adherence. Targeting social and supportive factors, other interventions aim to quickly troubleshoot problems that occur during CPAP treatment and provide regular feedback and encouragement from automated prompts, peers, or healthcare providers. Alternatively, more interactive interventions, designed in accordance with various cognitive and behavioural models, seek to modify motivation, confidence, goal setting behaviours, and other psychological constructs in an effort to improve CPAP adherence. Often, a combination of approaches is used (Aloia 2013; Bartlett 2013; Bouloukaki 2014; Hui 2000; Hwang 2017; Lewis 2006; Meurice 2007; Sawyer 2017; Sedkaoui 2015; Shapiro 2017; Smith 2006; Wang 2012). On the technological domain, modifications of delivery of airway pressure, such as the use of automatically titrating CPAP (auto‐CPAP), bi‐level PAP (BPAP) and humidification therapy, claim to decrease side effects in the upper airway due to cold and dry airflow and thus improve the comfort of treatment, but have not yielded convincing benefits in clinical trials to date (Smith 2009).
Why it is important to do this review
Despite the apparent efficacy of CPAP and its therapeutic benefits extending beyond amelioration of OSA symptoms to other functional and potential health outcomes, treatment adherence has been persistently low (Rotenberg 2016). Interventions directed at improving CPAP usage vary in methodology, complexity and effectiveness. Knowing what type of intervention is efficacious and potentially effective in clinical practice would guide clinicians and health authorities in developing services and guidelines aimed at improving treatment adherence. Since the last Cochrane Review, published in 2014 (Wozniak 2014), which assessed educational, supportive and behavioural interventions aimed at improving CPAP usage, a substantial number of new studies have been reported. This review updates the evidence.
Objectives
To assess the effectiveness of interventions that employ educational, supportive, behavioural, or mixed approaches to encourage adults who have been prescribed continuous positive airway pressure (CPAP) to use their devices.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, parallel‐controlled trials of any duration.
Types of participants
Participants were adults of either sex with a diagnosis of obstructive sleep apnoea (OSA) using a recognised sleep diagnostic tool giving an Oxygen Desaturation Index (ODI) of ≥ 5 per hour or an apnoea hypopnoea index (AHI) ≥ 5 per hour. Trials that explicitly recruited patients with central sleep apnoea were not eligible for inclusion.
Types of interventions
Intervention group
Any short‐term or sustained intervention aimed at encouraging uptake, acclimation, improvement or maintenance of continuous positive airway pressure (CPAP) adherence among individuals with a diagnosis of OSA. Examples of modalities that our review intended to capture include educational, supportive, group‐based, mindfulness‐based, cognitive, behavioural, motivational or approaches utilising a combination of these strategies.
Control group
Participants in the control group may receive instruction that would be used by the study centre in question, provided that the equivalent 'background' level of instruction was also offered and delivered to the intervention group. Intervention and control groups must have also either 1) received the same make of CPAP machine and pressure delivery mode (i.e. fixed, auto‐titrating, bi‐level PAP (BPAP), etc.) or 2) receive PAP devices in a randomly distributed manner, such that device make remained independent of group assignment.
Types of outcome measures
Primary outcomes
CPAP device usage (hours/night) as measured by:
microprocessor and monitor that measure pressure at the mask for every minute of each 24‐hour day;
counter output that records the cumulative time that power is turned on for a CPAP machine (this does not provide information on actual time of day and duration of CPAP used during each 24‐hour period);
subjective participant reports of the duration of CPAP use.
Secondary outcomes
The following secondary outcomes were considered:
proportion of participants adherent (≥ 4 hours/night);
sleepiness symptom scores such as the Epworth Sleepiness Scale (ESS);
disease‐specific quality of life scores such as Functional Outcomes of Sleep Questionnaire (FOSQ) or Calgary Sleep apnoea Quality of Life Index (SAQLI) scores;
any standardised depression or anxiety symptom scale measurement;
withdrawals from the study;
oxygen desaturation index (ODI), apnoea hypopnoea index (AHI);
cost‐effectiveness.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org)
Weekly searches of MEDLINE Ovid SP 1946 to April 2019
Weekly searches of Embase Ovid SP 1974 to April 2019
Monthly searches of PsycINFO Ovid SP 1967 to April 2019
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) 1937 to April 2019
Monthly searches of AMED EBSCO (Allied and Complementary Medicine) all years to April 2019
Hand searches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register were identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings are in Appendix 2. See Appendix 3 for search terms used to identify studies for this review.
Searches in the Cochrane Airways Trials Register and additional sources were completed from inception to April 2019, with no restrictions on language or publication type. Review authors attempted to contact authors to locate potential unpublished or in‐progress studies that met the inclusion criteria. When seeking further information of studies represented by trial registries or conference proceedings abstracts, the review authors contacted the trial authors for clarification if 1) the study was deemed to be potentially relevant according to inclusion criteria, 2) if the study appeared to be complete based on the information documented on ClinicalTrials.gov, 3) if no full publication was listed on the trial registry records, 4) and if no full publication was identified via an author/title/element PubMed search.
Searching other resources
We searched Epistemonikos (International database of systematic reviews) all years to April 2019 to identify other relevant systematic reviews, and checked their reference lists. We completed additional handsearching of the bibliographies of identified trials. The 2013‐2018 ATS and the 2013‐2018 European Respiratory Society (ERS) meeting abstracts were also handsearched for the current review update.
Data collection and analysis
Selection of studies
Review authors (KDA and LW) independently reviewed the titles, abstracts and citations identified through electronic searching to assess potential relevance for full review. Conflicting decisions on inclusion were resolved through discussion and consensus. Records eligible for full‐text review were scrutinised independently (KDA and LW) for inclusion based on a priori criteria for population, study design, intervention and outcome. Agreement was measured by simple agreement and conflicting decisions on study inclusion were resolved through discussion and consensus (KDA, LW, DRW, IS). See Figure 1 Study Flow Diagram. Reasons for study exclusion at the full‐text review stage were agreed upon by review authors (KDA, LW, DRW, IS) and recorded in the Characteristics of excluded studies table. For included studies, descriptive information for studies and study populations is presented in tabular form (Table 5; Table 6; Table 7;Table 8; Table 9; Table 10; Table 11).
1.
Study flow diagram.
1. Number screened, entered and completed.
| Study | N Screened | Entered | Completed | % Screened | % Entered |
| Aloia 2001 | NA | 12 | 12 | NA | 100 |
| Aloia 2013 | 339 | 227 | 183 | 54 | 81 |
| Bakker 2016 | 479 (only 2 of 4 treatment arms included in this review) | 83 | 78 | 16 | 94 |
| Bartlett 2013 | 294 | 206 | 177 | 60 | 86 |
| Basoglu 2011 | 246 | 133 | 133 | 54 | 100 |
| Bouloukaki 2014 | 5100 | 3100 | 2836 | 56 | 91 |
| Chen 2015 | 85 | 80 | 80 | 94 | 100 |
| Chervin 1997 | NA (75% of those approached agreed to participate) | 33 | 33 | NA | 100 |
| Dantas 2015 | 61 | 41 | 40 | 66 | 98 |
| DeMolles 2004 | NA | 30 | 30 | NA | 100 |
| Diaferia 2017 | NA | 49 | 49 | NA | 100 |
| Falcone 2014 | 533 | 206 | 161 | 30 | 78 |
| Fox 2012 | NA | 75 | 54 | NA | 72 |
| Hoet 2017 | 127 | 46 | 37 | 29 | 80 |
| Hoy 1999 | NA | 80 | 80 | NA | 100 |
| Hui 2000 | NA | 108 | 97 | NA | 90 |
| Hwang 2017 | 1873 | 1455 | 1236 | 66 | 85 |
| Lai 2014 | 212 | 100 | 98 | 46 | 98 |
| Lewis 2006 | 74 | 72 | 58 | 78 | 81 |
| Mendelson 2014 | 107 | 107 | 82 | 77 | 76 |
| Meurice 2007 | 133 | 112 | 112 | 84 | 100 |
| Munafo 2016 | 140 | 140 | 122 | 87 | 87 |
| Olsen 2012 | 132 | 106 | 94 | 71 | 89 |
| Parthasarathy 2013 | 49 | 39 | 37 | 76 | 95 |
| Pengo 2018 | NA | 112 | 85 | NA | 76 |
| Pepin 2019 | NA | 306 | 239 | NA | 78 |
| Richards 2007 | 109 | 100 | 96 | 88 | 96 |
| Roecklein 2010 | NA | 30 | 28 | NA | 93 |
| Sarac 2017 | 490 | 115 | 115 | 23 | 100 |
| Sawyer 2017 | 431 | 118 | 103 | 24 | 87 |
| Scala 2012 | NA | 28 | 28 | NA | 100 |
| Sedkaoui 2015 | 391 | 379 | 377 | 96 | 99 |
| Shapiro 2017 | NA | 66 | 65 | NA | 98 |
| Smith 2006 | NA | 19 | 19 | NA | 100 |
| Smith 2009 | NA | 97 | 73 | NA | 75 |
| Soares‐Pires 2013 | NA | 202 | 146 | NA | 72 |
| Sparrow 2010 | 423 | 250 | 222 | 52 | 89 |
| Stepnowsky 2007 | 91 | 45 | 40 | 44 | 89 |
| Stepnowsky 2013 | NA | 241 | 240 | NA | 99 |
| Turino 2017 | NA | 100 | 100 | NA | 100 |
| Wang 2012 | NA | 152 | 130 | NA | 86 |
2. Descriptive summaries: particpant characteristics, by intervention class.
| Variable | Behavioural (BEH) | Educational (EDU) | Supportive (SUP) | Mixed (MIX) |
| N (total randomised) | 989 | 1878 | 1962 | 5041 |
| Age in years (Mean, SD) | 56.44 (5.76) | 52.73 (4.68) | 53.94 (4.88) | 52.55 (5.46) |
| BMI (Mean, SD) | 32.31 (2.90) | 34.19 (3.51) | 33.19 (2.02) | 33.73 (2.80) |
| Sex (% female)* | 34.38 | 29.98 | 24.68 | 32.44 |
| AHI (Mean, SD) | 38.08 (9.04) | 39.72 (12.25) | 41.11 (10.52) | 38.82 (10.62) |
| ESS (Mean, SD) | 12.80 (4.02) | 11.27 (1.29) | 10.47 (1.50) | 12.53 (1.92) |
* Percentage female calculated based on studies reporting statistics on gender (those not reporting excluded from calculation).
3. Descriptive summaries: intervention characteristics, by intervention class.
| Intervention Details | Behavioural (BEH), (median, IQR) | Educational (EDU), (median, IQR) | Supportive (SUP), (median, IQR) | Mixed (MIX), (median, IQR) |
| Study duration (weeks) | 12 (12‐52) | 12 (6‐26) | 12 (12‐16) | 14 (12‐52) |
| Intervention duration (weeks) | 4 (2‐12) | 0 (0‐4.5)* | 12 (9‐13) | 12 (10‐25) |
| # of Intervention episodes | 3 (3‐14) | 2 (1‐6) | NR (MOST) | 7 (5‐10) |
| Contact time (minutes) | 90 (80‐240) | 21 (11‐105) | NR | 75 (33‐143) |
* Educational interventions that took place in a single participant interaction (e.g., dispensing written material, single presentation) were assigned a duration of '0' weeks.
Abbreviations: IQR: interquartile range; NR: not reported; NR (MOST): most studies did not report.
4. EDU Study characteristics.
| Study | Studies employing Educational Intervention | Control | Study duration (weeks) | |
| Increased support and reinforcement components (if applicable) | Increased educational components | |||
| Aloia 2013 | 2 x 45‐minute education sessions regarding pathophysiology of apnoea, medical and behavioral consequences, and the benefits of treatment; presented in standardised formats, with no tailoring to participant readiness, 1 booster call from sleep nurse | Usual care | 52 | |
| Basoglu 2011 | One 10‐minute educational video session on OSA and CPAP | Usual care | 24 | |
| Chervin 1997 | Written information on OSA and CPAP | Usual care | 8 | |
| Falcone 2014 | Two consecutive PSG videos on the computer screen: the first recorded during a standard diagnostic overnight polysomnography, and the second during a full‐night polysomnography with nasal CPAP | Usual care | 52 | |
| Hwang 2017 | Education about OSA pathophysiology , health‐related risks, impact on daytime vigilance, introduction to CPAP therapy | Usual care | 12 | |
| Pengo 2018 | Positively or negatively framed messages in addition to CPAP. Patients were phoned weekly and read framed messages (≤ 6 phone calls per patient). | Usual care | 6 | |
| Richards 2007 | Slide presentation and written information on OSA and CPAP and 2 x 1‐hour CBT sessions | Usual care | 4 | |
| Roecklein 2010 | Personalised feedback report, including detailed information OSA and its associated risk and barriers to CPAP use and attitudes to change | Usual care | 12 | |
| Sarac 2017 | 1 x 20‐minute educational session by a sleep medicine physician, including: viewing his/her own PSG chart on morning post PAP‐titration, comparing PSG from diagnostic and CPAP titration studies with explanations that emphasized obstructive events and oxygen desaturations, and the disappearance of those signs on PAP treatment. | Usual care | 24 | |
| Soares‐Pires 2013 | 1 x 1‐hour educational session with information regarding OSA, its symptoms and risks, APAP treatment, the importance of good adherence, and different machine interfaces. | Usual care | 24 | |
| Wang 2012 | Two additional nights of CPAP titration | 4‐hour group education session, written information, video CD | Usual care | 12 |
Abbreviations:
CBT: Cognitive behavioural therapy; CD: compact disc; CPAP: continuous positive air pressure; OSA: obstructive sleep apnoea; PAP: positive air pressure; PSG: polysomnography
5. SUP Study characteristics.
| Study | Studies employing Supportive Intervention | Control | Study duration (weeks) | |
| Increased support and reinforcement components | Increased educational components (if applicable) | |||
| Chervin 1997 | Weekly telephone calls to monitor progress and troubleshoot | Usual care | 8 | |
| DeMolles 2004 | Computer‐based telecommunication system allowing for monitoring and reinforcing compliance | Education via computer‐based telecommunication system | Usual care | 8 |
| Fox 2012 | Telecomunication system for daily monitoring of CPAP usage, timely detection and troubleshooting of problems | Usual care | 12 | |
| Hoet 2017 | Telemonitoring device forair leaks, residual AHI > 10/h, or CPAP use less than 3 hours for 3 days | Usual care | 12 | |
| Hoy 1999 | 2 additional titration nights in hospital, 4 additional home visits by sleep nurses | Initial education at home with partner | Usual care | 24 |
| Hwang 2017 | Automatic processing of device data. Where CPAP usage thresholds met, automated message encouraged participant to improve use/positive reinforcement | Usual care | 12 | |
| Mendelson 2014 | Participants equipped with smartphone for uploading BP, CPAP adherence, sleepiness, and QoL data. They received daily pictograms containing health‐related messages | Usualo care | 16 | |
| Munafo 2016 | Web‐based app used to monitor adherence and automatically message patients and providers when pre‐set conditions met | Usual care | 12 | |
| Parthasarathy 2013 | 2 individual sessions and 8 telephone conversations with trained peer CPAP users providing support and sharing their positive experience with CPAP | Usual care | 12 | |
| Pepin 2019 | BP and physical activity recorded by multimodal telemonitoring device and electronic questionnaires completed by patients. Automatic algorithms constructed for prompt adjustment of CPAP treatment. | Usual care | 24 | |
| Stepnowsky 2007 | Daily wireless telemonitoring of compliance and treatment efficacy and acting on the data via prespecified clinical pathways | Usual care | 8 | |
| Stepnowsky 2013 | Telemonitoring device collecting daily CPAP adherence viewable by both patient and provider. Troubleshooting and feedback provided when necessary | Usual care | 16 | |
| Turino 2017 | Daily CPAP adherence, CPAP pressures, mask leak and residual respiratory events transmitted into a web database. Case by case guidance provided by provider when signalled by automatic alarm in the web database | Usual care | 12 | |
Abbreviations:
AHI: apnoea hypopnoea index; BP: Blood pressure; CPAP: continuous positive air pressure; QoL: quality of life.
6. BEH Study characteristics.
| Study | Studies employing Behavioural Intervention | Control | Study duration (weeks) | ||
| Increased support and reinforcement components (if applicable) | Increased educational components (if applicable) | Behavioural therapy | |||
| Aloia 2001 | Elements of education on consequences of OSA and efficacy of CPAP | 2 x 45‐minute sessions of CBT interventions | 2 x 45‐minute sessions on sleep architecture and sleep clinic | 12 | |
| Aloia 2013 | 2 x 45‐minute sessions of MET, one booster phone call | Usual care | 52 | ||
| Bakker 2016 | Eight ‐ hour in person MET session | Usual care | 52 | ||
| Dantas 2015 | 1 x 10‐minute MET session | Usual care | 8 | ||
| Diaferia 2017 | Thirty‐six myofunctional therapy sessions | Usual care | 36 | ||
| Lai 2014 | One brief MET session (video and patient interview), followed by a follow‐up phone call | Usual Care | 12 | ||
| Olsen 2012 | 45‐Minute individual education session | Three 30‐minute sessions of MET | 45‐Minute educational session + usual care | 52 | |
| Scala 2012 | 3 interactive sessions, video with discussion, focus group and role play, respectively 1, 2 and 3 months after receiving the CPAP device. | Usual Care | 52 | ||
| Smith 2009 | Audiotaped music and softly spoken directions on relaxation techniques and habit‐promoting instructions for using CPAP nightly. Information packet,including CPAP use reminder placard, handouts on benefits of CPAP adherence and health consequences of poor compliance, 4‐week diary for recording experience with CPAP | Audiotaped music with softly spoken information on vitamins, informational packet on vitamins and health. | 12 | ||
| Sparrow 2010 | Automated telephone‐linked communication system designed around the concept of Motivational Interviewing, which allowed one to assess and enhance CPAP compliance | Education on unrelated health topics via automated telephone‐linked communication system | 52 | ||
| Wang 2012 | One night of CPAP titration in the hospital | 12 x 40‐minute group PMR practice sessions over 12 weeks, one per week. Self‐practice of PMR before each CPAP treatment. Brochure and CD with a guide for PMR practice at home. | Usual care | 12 | |
Abbreviations:
CBT: Cognitive behavioural therapy; CPAP: continuous positive air pressure; MET: Motivational Enhancement Therapy;OSA: obstructive sleep apnoea; PMR: progressive muscle relaxation;
7. MIX Study characteristics.
| Study | Studies employing Mixed Intervention | Control | Study duration (weeks) | ||
| Increased support and reinforcement components | Increased educational components | Behavioural therapy | |||
| Bartlett 2013 | 1 x 30 minute group education session | 1 x 35‐minute intervention based on SCT , including perceived self‐efficacy, outcome expectations, and social support | Usual care + a 30‐minute group education session and social period matching the duration of the intervention | 24 | |
| Bouloukaki 2014 | Two phone calls from study nurse to discuss CPAP use, 1 month of sleep diary review by sleep specialist, and 6 in‐person follow‐ups involving patient's family or spouse | 1 x 15 minute video education session covering OSA topics, followed by 10‐minute lecture to reinforce key topics | Usual care | 104 | |
| Chen 2015 | Personalised guidance from a study nurse, home visits from a nurse discussing lifestyle management, mental well‐being, and 1 x 30‐minute consultation with a sleep physician | 1 x pre‐treatment OSA educational video | Usual care | 52 | |
| Hui 2000 | 2 additional early reviews by sleep physician and frequent telephone calls by sleep nurses | Videotape and additional education session | Usual care | 12 | |
| Hwang 2017 | Intervention based on automatic processing of device data. If CPAP usage thresholds were met, a message was automatically sent to the patient providing encouragement to improve use or positively reinforcing successful adherence. | Education about pathophysiology of OSA, health‐related risks, impact on daytime vigilance, introduction to CPAP therapy | Usual care | 12 | |
| Lewis 2006 | 1 additional early review by sleep physician and 1 early telephone interview with sleep nurse | Educational video | Usual care | 52 | |
| Meurice 2007 | 4 additional home visits in the first 3 months by sleep practitioner for problem solving | Written information and detailed explanation by the prescriber, additional education during home visits | Written information and detailed explanation by the prescriber + usual care | 52 | |
| Sawyer 2017 | Educational DVD on sleep apnoea and PSG review | 4 x 30‐60 minute sessions addressing cognitive perceptions of the OSA and CPAP, outcome expectancies with PAP treatment, and PAP treatment self‐efficacy, all domains of SCT | Usual care and an informational pamphlet about OSA, diagnosis and PAP prescription provided by sleep centre | 12 | |
| Sedkaoui 2015 | 5 x standardised support sessions through telephone‐based counselling | Education addressing knowledge about OSA, disadvantage or obstacles to CPAP | Usual care | 16 | |
| Shapiro 2017 | 2 x support calls with study investigator to promote the use of CPAP | 1 x educational session using an airway model along with a video and worksheet on OSA, and a report card to document OSA severity, CPAP setting and use and participant self‐evaluation | Usual care | 4 | |
| Smith 2006 | Home video‐link sessions delivered by nurse, who guided correct CPAP use and provided problem solving | Nurse provided education on CPAP and OSA | Home video‐link sessions similar in form to intervention but directed activities in neutral health topics (vitamin intake) | 12 | |
| Wang 2012 | Three nights of CPAP titration in the hospital | 4‐hout group education session, written information, video CD | 12 x 40 minute group PMR practice sessions over 12 weeks | Usual care | 12 |
Abbreviations:
CPAP: continuous positive air pressure;DVD: Digital versatile disc; OSA: obstructive sleep apnoea; PAP: positive air pressure; PSG: polysomnography; SCT: social cognitive therapy
Data extraction and management
Data from published studies were extracted (KDA and TE) and checked (KDA, TE, LW) independently. Data were extracted first to an excel database and then to RevMan. After completion of RevMan data input from excel database, data in RevMan were checked for errors by comparison of RevMan tables to original published reports (LW). When data were unavailable from trial registries or conference abstracts, study authors were contacted (TE) to determine if data may be obtained directly. Information from authors was also sought to validate study design and methods for 'Risk of bias' and GRADE assessments. Manuscripts published in languages other than English were translated by volunteers co‐ordinated by Cochrane Airway's Assitant Managing Editor using a standardised form.
Categorisation of studies
In an attempt to limit the heterogeneity that arises when studies are combined into one overarching comparison, studies were classified into one of four comparisons based on the prevailing nature of the active intervention. Classifications were determined by detailed review of study authors' intervention descriptions, rather than the label applied to the intervention by study authors (e.g. in title or abstract). In most cases, the authors' designation was consistent with our judgement.
Educational versus control – Interventions imparting information about CPAP treatment or about OSA more generally, delivered through face‐to‐face didactic sessions, group educational sessions, written materials, video format, or any combination of these. Interventions that did not involve a component of active engagement from the participants other than reading written materials or observing a presentation or demonstration, even if the content derived from a behavioural change model, were classified as educational.
Supportive versus control ‐ Interventions in which participants were provided with additional clinical follow‐up (e.g. additional office‐ or home‐based visits or phone check‐ins by clinical staff), or with telemonitoring equipment that facilitated either self‐monitoring of CPAP usage or monitoring by clinical staff to prompt 'as needed' clinical follow‐up (e.g. a phone call made to participants when CPAP usage fell below a predetermined threshold) for the purpose of addressing barriers or difficulties with CPAP usage in a timely manner (e.g. telemedicine systems, digitised phone calls or audio messages, and home visits). Thus, supportive interventions either encouraged participants to provide feedback on their experience of CPAP treatment on an ongoing basis or employed automated assessment of transmitted CPAP data to trigger clinician review/intervention.
Behavioural versus control ‐ Interventions employing psychotherapeutic techniques deriving from behavioural, cognitive or related models of health behaviour change (e.g. specific models within this broad genre include motivational enhancement therapy (Miller 1994), Social Cognitive Theory (Bandura 1982; Bandura 1986), Transtheoretical/Stages of Change Model (Prochaska 1983), and cognitive behavioural therapy (CBT) (Beck 1975)). By definition, behavioural interventions under any of these related models involves at least a minimal degree of direct participant engagement or interaction (as opposed to purely educational, in which information is merely imparted to participants, even if the educational content or style of presentation was based on a behavioural model). Thus, behavioural interventions targeted a modifiable and measurable construct known or hypothesised to influence health beliefs about OSA and CPAP therapy and CPAP adherence behaviour. The objectives of such interventions might include enhancing behavioural action, motivation for change, self‐efficacy, outcome expectations and decisional balance in favour of CPAP.
Mixed versus control – Interventions that combined elements of two or more previously listed intervention‐types (e.g. educational video + telemedicine follow‐up), and therefore met criteria for belonging to more than one of the above‐described classes.
For studies that employed multiple intervention arms, the active interventions arms were separated and included in the appropriate comparison class depending exclusively on the content of that arm (i.e. each arm was independently classified as educational, supportive, behavioural or mixed).
Assessment of risk of bias in included studies
The review authors (KDA, LW, TE) assessed the risk of bias of included studies for the primary outcome, CPAP usage, according to the revised Cochrane 'Risk of bias 2' tool (Sterne 2019) Cochrane's recommended 'Risk of bias' tool for randomised trials as of 15 March 2019, which includes the following five domains:
randomisation processes;
deviations from intended interventions;
missing outcome data;
measurement of outcome;
selective outcome reporting.
Following detailed guidance provided in the revised Cochrane 'Risk of bias 2' full guidance document (Higgins 2019) and utilising the 'Risk of bias 2' excel tool (downloaded 04 April 2019), we graded each potential source of bias as 'low', 'some concerns', or 'high' and provided justification for item‐ and domain‐level judgements.
Given the nature of interventions, we did not anticipate blinding of participants in studies; however we attempted to determine if data collectors and analysts were blinded until the end of study data collection for 'Risk of bias' assessment.
Review authors (KDA, LW, TE) used the 'Risk of bias2' tool to perform additional 'Risk of bias' assessments as part of GRADE assessments. See Data collection and analysis section, 'Summary of findings' sub‐section for details of GRADE 'Risk of bias' assessments.
We used the 'Risk of bias1' tool to provide study‐level judgement for each new study under the following domains: random sequence generation; allocation concealment; blinding (performance and detection bias); incomplete outcome data; selective outcome reporting; other bias.Studies included in the previous review update were independently assessed by two review authors (DRW, IS) using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and included in the previous report. Studies new to this review update were independently assessed by two review authors (LW, TE) using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and added to the previous assessments. We graded each potential source of bias as high, low, or unclear and provided our rationale based on information from the study report and our judgement.
Measures of treatment effect
Effect measures
For dichotomous outcomes, an odds ratio (OR) and 95% confidence intervals (CIs) were calculated on the basis of the number of participants with an event versus the number of participants without an event. Mean differences (MDs) and 95% CIs were calculated for continuous variables in which all studies employed the same outcome measure or instrument (e.g. CPAP usage measured by device; sleepiness measured by ESS). Standardised mean differences (SMDs) were calculated for continuous variables in which studies employed different outcome measures or instruments (e.g. Functional Outcomes of Sleep Questionnaire (FOSQ) and Calgary Sleep apnoea Quality of Life Index (SAQLI) as metrics for quality of life).
Handling skewed data
Our protocol specified that when either medians or interquartile ranges were reported for treatment effects, this would serve as an indicator of skewed outcome distributions. In these cases, analysis based on means were not possible or appropriate. For outcomes where the lowest or highest possible value is known to exist (and not for values such as change from baseline measures), we planned to conduct a rough check for skew as follows: The observed mean would be subtracted from the highest possible (known) value (or the lowest possible (known) value subtracted from the observed mean), and this quantity would be divided by the standard deviation (SD). If the resulting ratio was < 2, this would suggest skew and a ratio < 1 would be considered strong evidence of a skewed distribution. In cases of strong evidence of skew, we would seek to collect appropriate data from the trialists. Appropriate data summaries and analytic strategies would depend on the situation and consultation with a knowledgeable statistician would be sought when necessary.
Unit of analysis issues
The unit of analysis was the patient.
Studies with multiple treatment groups
In most cases, intervention arms of multiple‐arm studies fell under distinct intervention classes (e.g. one arm was educational and another behavioural). In such cases (Aloia 2013; Chervin 1997; Hwang 2017; Wang 2012), the intervention arms were considered within their appropriate class and the full control arm was included in each class‐specific comparisons.
For multiple‐arm studies in which separate intervention arms fell under the same class (Meurice 2007; Pengo 2018), all relevant experimental arms were combined to conduct a single pair‐wise comparison to the full control arm (Higgins 2011).
Dealing with missing data
Where studies had missing data (e.g. means reported without SD), we contacted trial authors by email with a request for complete data. Data that were still missing after efforts to secure it were handled as follows. Data assessed to be missing at random are unlikely to bias results, so analysis proceeded with available data in those instances. For data determined to be not missing at random, an imputation strategy that accounts for uncertainty in the imputed values and results was employed. Per our protocol, sensitivity analyses were conducted if necessary to determine the potential impact of these assumptions.
Assessment of heterogeneity
We used the Inconsistency statistic (I2) to measure heterogeneity among the trials in each outcome analysis. For outcomes without evidence of heterogeneity (I2 = 0%) a fixed‐effect model was used. For outcomes with non‐zero measures of inconsistency (I2 > 0%), potential sources of heterogeneity were explored, including examination of small‐study effects and differences in magnitude or direction of effect. In outcomes for which heterogeneity was explained after examination, the nature of the explanations uncovered were used to make decisions regarding whether to proceed with the meta‐analysis of that outcome, the analysis model to be used, and whether further sensitivity analyses were warranted (Higgins 2011). In outcomes for which heterogeneity remained unexplained but meta‐analysis still warranted, we employed a random‐effects model.
Assessment of reporting biases
We assessed publication bias using funnel plot estimate when criteria to apply asymmetry tests were met (i.e. ≥10 studies in the outcome, heterogeneity I2≤ 50%, ratio of maximal to minimal variance across studies > 4 (Ioannidis 2017). When these criteria were not met, assessment of publication bias was based on guidelines provided in the GRADE handbook, Section 5.2.5 Publication Bias, and published online tutorials provided by Cochrane and GRADEpro online software (Schunemann 2013; GRADEpro 2015)
Data synthesis
See Measures of treatment effect section for description of the effect measures used by review authors to describe effect sizes in included studies and meta‐analyses. Results were combined across studies for meta‐analysis, subgroup and sensitivity analyses using RevMan software. Heterogeneity assessment was also conducted in RevMan for each comparison.
Subgroup analysis and investigation of heterogeneity
Subgroup comparisons planned a priori included the following.
Participants with prior CPAP exposure versus CPAP‐naive participants
Sex (male versus female)
Baseline AHI: mild (AHI ≥ 5 to < 15), moderate (AHI ≥ 15 to < 30), severe (AHI ≥ 30)
Baseline Epworth Sleepiness Scale Score (ESS: 0 to 10 versus 11 to 24)
Sensitivity analysis
For our main outcome of CPAP usage, we planned (a priori) sensitivity analyses to analyse studies in which CPAP usage in the control arm was < 4 hours per night and studies in which participants were unaware that their CPAP usage was being recorded.
'Summary of findings' tables
We included 'Summary of findings' tables for the four comparison categories (behavioural versus control, educational versus control, supportive versus control, mixed versus control). Information about the following key outcomes is presented in the tables where possible.
CPAP machine usage
Sleepiness, depressive and anxiety symptoms
Quality of life
Study withdrawal
Cost‐effectiveness
We additionally applied methods outlined by the GRADE working group (Schunemann 2013; GRADEpro 2015) to rate the confidence in estimates by considering the following domains.
Risk of bias
Imprecision
Inconsistency
Indirectness
Publication bias
Large effects
In downgrading risk of bias within GRADE assessments, we followed the guidance provided in the GRADE Handbook section on guidelines for authors of systematic reviews. When assessing a group of studies (e.g. within an intervention class), risk of bias was downgraded by one level if the combined weight of studies with high risk of bias was > 50%. It was downgraded by an additional level if, in addition, the remaining studies had 'Risk of bias' ratings that were predominantly ‘some concerns’.
Results
Description of studies
Results of the search
See Figure 1 for the study flow diagram. From the previous update of this review, we retained 25 studies (literature search dates: all years to January 2013). Five previously included studies were excluded in the present review for the following reasons: study analysed wrong outcomes (Schiefelbein 2005), not all inclusion criteria met (Taylor 2006; Wiese 2005), no full published report currently available (Epstein 2000), and study record was a duplicate entry for a published report (NCT01715194). Updated searches conducted to May 2019 yielded 16 new studies that met the review's inclusion criteria.
This review summarises the evidence from all 41 included studies. For descriptions of each study, refer to the Characteristics of included studies of this paper. Forty‐seven additional studies were judged to be potentially relevant but could not be assessed for inclusion until additional information is obtained; these were assigned to Studies awaiting classification. Six additional studies were identified as relevant but are currently ongoing (i.e. data and results are not yet publicly available) and were therefore assigned to Ongoing studies (Abreu 2013; Bakker 2017; Castronovo 2017; Crawford 2016; Kotzian 2018; Seixas 2018).
We excluded 101 studies from this review; please see Characteristics of excluded studies for reasons for exclusion.
Included studies
Study design
All studies were randomised, single‐blind or unblinded parallel‐group studies.
Participants
A total of 9005 participants were included and randomised in the studies (Table 5). Mean (SD) age across all studies was 54.3 (5.3). Mean of apnoea hypopnoea index (AHI), Epworth Sleepiness Scale (ESS) and body mass index (BMI) across studies reporting these values was 40.6 (9.9), 11.9 (2.4) and 33.1 (2.7), respectively. Among included studies reporting ESS at baseline, average ESS scores at baseline indicated that 75% of participants suffered from excessive daytime somnolence (ESS 11 to 24). Overall mean baseline AHI among included studies reporting AHI was 40.6 (median 40.7) events/hour, corresponding to severe obstructive sleep apnoea (OSA). Additionally, average AHI measurements at baseline indicated that 82% of participants had severe OSA (AHI > 30). Twenty‐seven studies included participants that were naive to continuous positive airway pressure (CPAP) therapy; 13 did not specify CPAP naivete, and one study included participants who were previously exposed to CPAP therapy. See Table 6 for a breakdown of mean participant characteristics by intervention class.
Included randomised controlled trials (RCTs) were conducted between 1997 and 2018 in 14 countries: Australia (3), Belgium (1), Brazil (1), Canada (1), China (2), Hong Kong (2), France (4), Greece (1), Italy (2), Portugal (2), Spain (1), Turkey (2), UK (2), Scotland (1), and USA (16).
Sample sizes ranged from 12 (Aloia 2001) to 3100 (Bouloukaki 2014). Most studies included in this review were small: 17 studies randomised < 100 participants, 13 randomised 100 to 199 participants, and 11 randomised > 200 participants.
Gender distribution ranged from 0% (Diaferia 2017; Parthasarathy 2013) to 75.3% (Scala 2012) female, with a mean of 28.42% female. Gender distribution was not reported in five studies, but it is likely that these studies were 100% male, both because gender was not reported and because many appear to have been conducted in Vetarans Affairs (VA) hospitals or by investigators with primary VA affiliation.
The majority of study authors did not report outcomes segregated by sex, baseline AHI or baseline ESS. Thus, while our protocol pre‐specified possible subgroup analyses on these bases, there were insufficient data available for such analyses. Additionally, the vast majority of studies recruited participants with newly‐diagnosed OSA or obstructive sleep apnoea syndrome (OSAS), and a small number either included participants with previous diagnosis or did not report whether participants with previous treatment were excluded. Thus, subgroup analysis on the basis of prior CPAP treatment was also not performed.
Interventions
All included studies were classified into one of four types: educational, supportive, behavioural, and mixed intervention. In cases where a study would qualify for classification in more than one class, the study was classified as mixed. For a quick overview of included studies (and their active components) by intervention class, refer to Table 8 (Educational); Table 9 (Supportive); Table 10 (Behavioural); Table 11 (Mixed). Among studies with multiple active intervention arms, each arm was classified separately and included in the respective class meta‐analysis. More detailed descriptions of our classification are provided in Data collection and analysis, Data extraction and management.
Educational interventions were delivered using a variety of techniques, including educational/situational videos (Basoglu 2011; Richards 2007), group education sessions (Soares‐Pires 2013), extended and personalised explanation of polysomnography (PSG) reports (Falcone 2014; Roecklein 2010; Sarac 2017), and positive/negative risk message framing (Pengo 2018).
Supportive interventions included telemonitoring under various formats and platforms (DeMolles 2004; Fox 2012; Hoet 2017; Mendelson 2014; Munafo 2016; Pepin 2019; Stepnowsky 2007; Turino 2017), in‐home tutorials and extended follow‐up visits (Hoy 1999), peer support (Parthasarathy 2013), phone support (Chervin 1997) and personalised web‐based support platforms (Stepnowsky 2013).
Various strategies were employed across included behavioural studies, including Motivational Enhancement Therapy (MET) aimed at resolving ambivalence towards treatment (Aloia 2001; Bakker 2016; Lai 2014; Olsen 2012; Sparrow 2010), a combination of various motivational strategies (Dantas 2015; Scala 2012), habit‐promoting audiotapes (Smith 2009), and myofunctional therapy (Diaferia 2017).
The majority of studies in the mixed class used a combination of educational materials (videos, brochures, tutorials) and support systems (telemedicine or extended follow‐up) in their active intervention (Bouloukaki 2014; Chen 2015; Hui 2000; Hwang 2017; Lewis 2006; Meurice 2007; Sedkaoui 2015; Shapiro 2017 ). Other studies incorporated behavioural with educational intervention components (Bartlett 2013; Wang 2012), behavioural and supportive (Smith 2006), or components from all three classes (Sawyer 2017).
Detailed information pertaining to control interventions can be found in Characteristics of included studies.
Study duration, number of intervention episodes, total contact time
Total study duration varied greatly: four weeks (Richards 2007; Shapiro 2017), six weeks (Pengo 2018), two months (Dantas 2015; Chervin 1997; DeMolles 2004; Stepnowsky 2007), three months (Aloia 2001; Diaferia 2017; Fox 2012; Hoet 2017; Hui 2000; Hwang 2017; Lai 2014; Munafo 2016; Parthasarathy 2013; Roecklein 2010; Sawyer 2017; Smith 2006; Smith 2009; Turino 2017; Wang 2012), four months (Mendelson 2014; Sedkaoui 2015; Stepnowsky 2013), six months (Bartlett 2013; Basoglu 2011; Hoy 1999; Pepin 2019; Sarac 2017; Soares‐Pires 2013), 12 months (Aloia 2013; Bakker 2016; Chen 2015; Falcone 2014; Lewis 2006; Meurice 2007; Olsen 2012; Scala 2012; Sparrow 2010), and 24 months (Bouloukaki 2014). The number of intervention episodes (i.e. number of discrete episodes of contact with study personnel) among studies specifying varied from one (Bartlett 2013; Basoglu 2011; Dantas 2015; Falcone 2014; Roecklein 2010; Soares‐Pires 2013) to 36 (Diaferia 2017). The total intervention contact time was not specified for many studies (DeMolles 2004; Fox 2012; Hoet 2017; Hoy 1999; Hwang 2017; Mendelson 2014; Meurice 2007; Munafo 2016; Parthasarathy 2013; Pepin 2019; Roecklein 2010; Scala 2012; Sparrow 2010; Stepnowsky 2007; Stepnowsky 2013; Turino 2017); among the 25 studies reporting such information, contact time varied from five (Falcone 2014) to 720 (Diaferia 2017) minutes. See Table 7 for summary descriptions of these intervention characteristics by intervention class.
Outcomes
The majority of studies reported hours of machine usage at one or more time points, with the exception of three studies (Basoglu 2011; Smith 2006; ) who reported only proportions of participants who were adherent (yes/no) based on authors' pre‐determined threshold definition. The majority of studies also reported ESS data and participant withdrawals. A subset of studies reported quality of life using a variety of measurement instruments (Bartlett 2013; Bouloukaki 2014; Chen 2015; DeMolles 2004; Hoy 1999; Hwang 2017; Lai 2014; Mendelson 2014; Meurice 2007; Parthasarathy 2013; Pepin 2019; Scala 2012; Stepnowsky 2007; Stepnowsky 2013), depressive or anxiety symptom ratings using a variety of measurement instruments (Bartlett 2013; Bouloukaki 2014; Chen 2015; Hoy 1999; Shapiro 2017; Stepnowsky 2007; Stepnowsky 2013; Wang 2012) oxygen desaturation index (ODI)/AHI measurements (Dantas 2015; Diaferia 2017; Fox 2012; Stepnowsky 2007), and cost‐effectiveness (Bouloukaki 2014; Turino 2017). Finally, Bouloukaki 2014 reported average hours of CPAP usage per night used, rather than the standard values of average hours used per night, overall. Calculations based on per night used would result in potentially significant upward bias in mean usage values relative to studies reporting average use per total intervention time period. However, since the same statistics are reported for intervention and control arms, the mean differences may not be biased. Bouloukaki 2014 data were included in CPAP usage meta‐analysis despite this discrepancy.
Endpoints reported
Due to the tremendous variability and difficulty in interpreting meta‐analytic results for temporally‐disparate endpoints, we elected to use an endpoint of three months (or the measured endpoint closest to three months), which was both the modal endpoint across studies and the most clinically‐relevant among those commonly reported.
Outcomes: exclusion of specific studies from selected meta‐analyses
Sparrow 2010 met our inclusion criteria, however, we were not able to include this study in our primary CPAP usage meta‐analysis because trialists presented their results as a mean difference (MD) and 95% confidence intervals (CIs) derived from a regression of log‐transformed CPAP usage data, and could therefore not be combined with data from the other studies (note: analysis by generic inverse variance (GIV) was also not suitable). Nonetheless, the direction of effect supports the general findings of Analysis 3.1 (CPAP usage 2.40 hours/night in intervention arm (N = 110) and CPAP usage 1.48 hours/night (N = 112) in the control arm). In Sparrow 2010, data were suitable for inclusion in meta‐analyses of other outcomes (N deemed adherent, withdrawal).
3.1. Analysis.
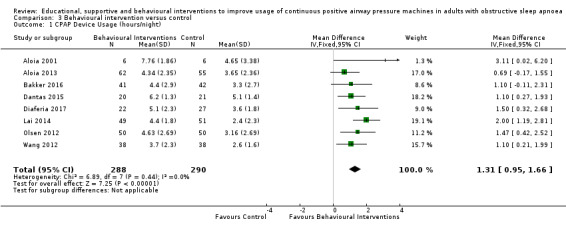
Comparison 3 Behavioural intervention versus control, Outcome 1 CPAP Device Usage (hours/night).
Lewis 2006 met our inclusion criteria and available data are shown in some outcome tables (e.g. Analysis 6.10). However, no SDs were available for reported mean CPAP usage and review authors were unable to obtain these data from the trial authors, so MDs could not be calculated. Therefore, this study was retained in the analysis table but SD values are entered as zero. Thus, it is excluded from the meta‐analysis of this outcome. Lewis 2006 data were suitable for inclusion in withdrawal meta‐analysis.
6.10. Analysis.
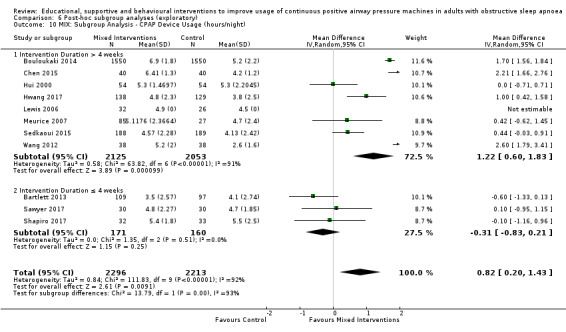
Comparison 6 Post‐hoc subgroup analyses (exploratory), Outcome 10 MIX: Subgroup Analysis ‐ CPAP Device Usage (hours/night).
Scala 2012 met our inclusion criteria, but CPAP usage data were not included in our analysis tables, or in our meta‐analysis because the data provided in the published report contained discrepancies that could not be resolved (i.e. reported mean, SD and P values were incompatible and review authors were unable to determine which values were incorrect). Scala 2012 was included in meta‐analyses for other outcomes (withdrawal, ESS and quality of life (QoL)).
Soares‐Pires 2013 was excluded from CPAP usage meta‐analysis because trial authors reported medians only. Review authors were unable to obtain information from authors necessary to implement planned skewed data handling procedures. Therefore, this study was excluded from our analysis tables and the meta‐analysis of this outcome. Soares‐Pires 2013 was included in meta‐analyses for other outcomes (N deemed adherent, withdrawal).
Excluded studies
We excluded 101 studies from this review. Reasons for their failure to meet review entry criteria are provided in Characteristics of excluded studies.
Risk of bias in included studies
It should be noted that full 'Risk of bias' assessments (evaluating each 'Risk of bias 2' domain using 'Risk of bias 2' tool) were performed for primary outcome (CPAP device usage, hours/night) only. Overall 'Risk of bias' assessments were additionally conducted for other outcomes as part of the GRADE assessment process, but the GRADE 'Risk of bias' assessment procedures were often more limited than that involved in the application of the full 'Risk of bias 2' tool, as the GRADE tool is outcome‐ (and not study‐) specific. Therefore, our procedures for GRADE risk of bias varied by outcome, as follows.
GRADE 'Risk of bias' judgements for CPAP usage and N deemed adherent were based on our 'Risk of bias 2' assessment judgements.
GRADE 'Risk of bias' ratings for all subjective, non‐adherence outcomes (i.e. ESS, QoL, depressive symptoms, anxiety symptoms) were judged to be 'serious' or 'very serious' because all, or nearly all, included studies had no masking (of participants or investigators) and such subjective/observational measures would be subject to measurement bias without blinding. Therefore, for these outcomes, domain 4 would be rated as 'high' risk of bias for all studies and result in a GRADE 'Risk of bias' rating of 'high' without the need to evaluate the other domains.
For the remaining outcomes (AHI, study withdrawal, cost‐effectiveness) GRADE 'Risk of bias' ratings were made based on examination of all 'Risk of bias 2' domains unless a 'high' rating was evident based on a preponderance (by weight) of 'high' ratings in study‐level 'Risk of bias 2' domains 1 or 2. (Because 'Risk of bias 2' domains 1 and 2 are study‐ and not outcome‐dependent, judgements of risk for domains 1 and 2 would be the same for all outcomes for a given study). The only outcome for which a high rating was not evident based upon a preponderance of high ratings in 'Risk of bias 2' domains 1 or 2 was 'study withdrawals' within supportive, behavioural and mixed classes. Thus, all 'Risk of bias 2' domains were evaluated for study withdrawals in these classes. Based on the definition of 'withdrawals' employed in our review (see Effects of interventions: Secondary outcomes, Withdrawals, below), 'Risk of bias 2' domain 3 (missing outcome data), domain 4 (bias in outcome measurement), and domain 5 (selective reporting) yielded the same judgement as for our primary outcome, CPAP usage. Because withdrawal was based on the absence of CPAP usage data, the proportions of participants withdrawing from the study would be the same as the proportion with missing CPAP usage data. Similarly, because our definition of withdrawal was based on objective data acquired from the CPAP device (i.e. either a zero usage value transmitted wirelessly or a device in the possession of study personnel and clearly not being used by the participant), judgement regarding the potential for measurement bias or selective reporting bias was the same as that rendered for the CPAP usage outcome in this domain.
Risk of bias 2: CPAP device usage
Risk of bias in included studies
An overview of our 'Risk of bias' judgements for our primary outcome for included studies (randomisation processes, deviations from intended interventions, missing outcome data, measurement of outcome, selective outcome reporting) is provided in Figure 2. The basis for each of these judgements is given in Characteristics of included studies. All of the studies were assessed according to assignment to intervention (the 'intention‐to‐treat' effect) using the 'Risk of bias 2' tool and according to the risk posed on measuring our primary outcome of hourly CPAP device usage. Half of the studies presented as having a high overall risk of bias.
2.
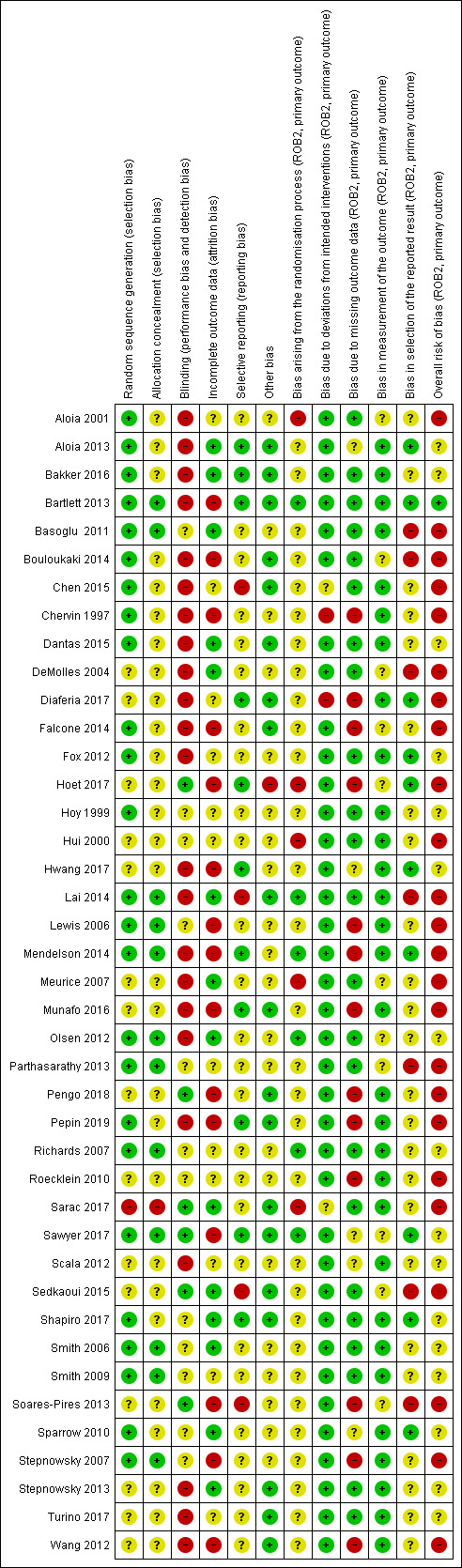
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Randomisation processes
The majority of studies presented as having "some concerns" regarding risk of bias arising from randomisation procedures; this was mostly due to inadequate descriptions of allocation sequence generation and allocation sequence concealment. Despite this, only a handful of studies presented significant baseline differences in key demographic characteristics (age, sex, BMI, and AHI), suggesting a potential problem with the study's randomisation procedures (Aloia 2001; Hoet 2017; Hui 2000; Meurice 2007; Sarac 2017). Six studies present low risk of bias in this domain (Bartlett 2013; Lai 2014; Mendelson 2014; Olsen 2012; Richards 2007; Sawyer 2017).
Deviations from intended interventions
Two studies presented "high" risk of bias in this domain (Chervin 1997; Diaferia 2017) due to the combination of the following factors: participants and trialists were aware of group assignment, a 'per‐protocol' analysis was employed (participants were analysed according to the treatment they received instead of the treatment to which they were randomised). Three studies presented as having "some concerns" regarding risk of bias in this domain (Chen 2015; Sarac 2017; Scala 2012). This was either due to inadequate information regarding the intervention group to which study "drop‐outs" belonged (i.e. only overall withdrawals were reported) (Chen 2015; Scala 2012) or potential deviations from the study protocol (Sarac 2017 ‐ phone call reminders were given to participants who did not show up for follow‐up appointments, regardless of group assignment).
Missing outcome data
Thirteen studies presented "high" risk of bias in this domain (Chervin 1997; Diaferia 2017; Falcone 2014; Hoet 2017; Lewis 2006; Mendelson 2014; Munafo 2016; Pengo 2018; Pepin 2019; Roecklein 2010; Soares‐Pires 2013; Stepnowsky 2007; Wang 2012), largely due to data being unavailable for ≥10% of participants at the time point analysed (Higgins 2011). Risk increased when considerable differences in proportions of missing outcome data between intervention and control groups were detected, indicating that loss to follow‐up was likely to be related to participant health status.
Measurement of the outcome
The majority of studies presented "low" risk of bias in this domain. Signalling questions addressed appropriateness of outcome measurement, differences in measurements between study groups, and whether outcome assessors were aware of group assignment. All of the studies included in this review measured hourly CPAP machine usage through an internalised digital counter or data microchip, thereby limiting the risk that could arise in this domain. Eleven studies did show "some concerns" (Aloia 2001; Bouloukaki 2014; DeMolles 2004; Falcone 2014; Hoet 2017; Meurice 2007; Olsen 2012; Parthasarathy 2013; Sawyer 2017; Sedkaoui 2015; Soares‐Pires 2013) as the review authors were unable to confirm with trialists if the distribution of CPAP devices (i.e. makes, models) differed significantly between groups.
Selective outcome reporting
Twenty‐three studies presented as having "some concerns" regarding risk of bias arising from selection of reported outcome. This was largely due to limited availability of pre‐specified study protocols or analysis plans, preventing review authors from comparing with published manuscripts to confirm that outcomes and outcome end points were determined prior to data analysis. Moreover, seven studies presented a "high" risk in this domain as a result of: using adherence thresholds or end points that are not commonly reported in literature, e.g. ≥ 3 hours per night instead of ≥ 4 hours per night, reporting at four months instead of three months (Basoglu 2011; Sedkaoui 2015), differences between intended end points in the 'Methods and Results' section of paper (Chen 2015; DeMolles 2004), modification of end points from that originally‐reported in study's trial registry archive (Lai 2014), reporting of adherence only in graphical format (NCT03345524), or reporting mean usage per effective days instead of per total days resulting in an upward bias of estimates (Soares‐Pires 2013; Bouloukaki 2014). See 'Risk of bias' tables for further details on our judgement rationale.
Risk of bias 1: study‐level 'Risk of bias' assessments
The review authors assessed the risk of bias of included studies using the risk of bias tool (Higgins 2011) (for an overall snapshot of our judgements, see Figure 2).
Allocation
The majority of studies (59%) were assessed as having low risk of bias for random sequence generation. Only one study (Sarac 2017) was found to have high risk of bias because, according to author's report, "participants were randomly assigned in order of appearance (random number table...) with exception for patients scheduled for weekend treatment, who were included in the [standard support] group." A judgement of 'Unclear' risk of bias under this domain was generally rendered because authors provided no or insufficient information regarding the random component used in sequence generation or other information regarding how randomisation was achieved.
The vast majority of studies were assessed as having unclear (68%) or low (29%) risk of bias for allocation concealment. One study (Sarac 2017) was found to have high risk of bias because the report permitted confirmation that allocation sequence was not concealed. Those determined to have unclear risk of bias under this domain were most often because the trialists provided no, or insufficient, information to permit assessment of allocation concealment method.
Blinding
The vast majority of studies were assessed as having high (56%) or unclear (29%) risk of bias in this domain because the majority of studies had at least one subjective outcome. Given the nature of the intervention, it is unlikely that blinding of participants is achievable and the majority of studies did not attempt to do so. Six studies (Hoet 2017; Pengo 2018; Sarac 2017; Sawyer 2017; Sedkaoui 2015; Soares‐Pires 2013) were assessed as having low risk of bias in this domain because these studies had only objectively‐measured outcomes, so a lack of blinding would unlikely affect those outcomes.
Incomplete outcome data
For this domain, 39% of studies were assessed as having high risk of bias, primarily due to either a very substantial proportion (> 10%) of participants with missing data and a substantial imbalance in missing across outcome classes. A judgement of 'Unclear' risk of bias (29%) in this domain was most often due to the absence of sufficient information to determine proportion of withdrawals or to compare class‐specific withdrawal rates. Data were available for all or nearly all randomised participants in 32% of studies, warranting a judgement of low risk of bias in this domain.
Selective reporting
For this domain, 63% of studies had 'Unclear' risk of bias primarily because no protocol or ClinicalTrials.gov entry was available to determine if analysis plan was finalised before unblinded outcome data were available for analysis. Those assessed to have 'low' risk of bias (27%) in this domain had a protocol, ClinicalTrials.gov entry or early abstract that permitted adequate verification that the analysis plan was finalised prior to analysis of unblinded data. For those studies judged to be at 'high' risk of bias, review authors found evidence that the outcomes reported were inconsistent with the methods section of the published report, were changed from those originally planned without clear rationale or were atypical (e.g. endpoint measurement, threshold cut‐off) without clear rationale, suggesting that the reported outcome may have been selected after analyses performed.
Other potential sources of bias
Each study was assessed for other potential risks of bias including deviations from intended interventions and baseline imbalances in important demographic or clinical characteristics (age, sex, BMI, AHI) across outcome classes. Reasons for a judgement of 'high' risk of Other bias (7%) were: failure to report selected baseline gender (Aloia 2013; Meurice 2007) and BMI data (Aloia 2013), and substantial baseline differences in gender distribution that was large enough to result in biased effect estimation (Hoet 2017). Studies for which a judgement of 'Unclear' was made were those that did not report baseline data or did not report a statistical comparison for important baseline characteristics across outcome classes.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Educational intervention versus control.
| Educational interventions + CPAP compared to usual care + CPAP in adults with obstructive sleep apnoea | ||||||
| Patient or population: adults with obstructive sleep apnoea Setting: community Intervention: educational interventions + CPAP Comparison: usual care + CPAP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care + CPAP | Risk with Educational interventions + CPAP | |||||
| 1.1 CPAP device usage (hours/night) | The mean CPAP device usage ranged from 1.97 to 5.1 hours/night | MD 0.85 hours/night higher (0.32 higher to 1.39 higher) | ‐ | 1128 (10 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | |
| 1.2 CPAP device usage (hours/night), sensitivity analysis: adherence in control group < four hours/night | The mean CPAP device usage , sensitivity analysis: adherence in control group < four hours/night ranged from 1.97 to 3.8 hours/night | MD 0.85 hours/night higher (0.06 higher to 1.64 higher) | ‐ | 698 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 5 6 | |
| 1.3 N deemed adherent (≥ four hours/night) | 558 per 1,000 | 765 per 1,000 (654 to 849) | OR 2.58 (1.50 to 4.44) | 1019 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 7 8 | |
| 1.4 Withdrawal ‐ NO META‐ANALYSIS PERFORMED | ‐ | 1745 (9 studies) | ‐ | |||
| 1.5 ESS ‐ Comparison of values at endpoint‐ NO META‐ANALYSIS PERFORMED | ‐ | ‐ | 355 (3 studies) | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CPAP: Continuous positive airways pressure;ESS: Epworth Sleepiness Scale; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; MD: mean difference; OR: Odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Overall risk of bias for this comparison was 'High' for 7/10 and 'some concerns' for the remaining 3/10. In those with high risk, risk derived from randomisation (1), missing outcome data (5), protocol deviation (1) and selective reporting (1). The combined weight of the studies with high risk is 59.2%. Therefore, risk of bias for this comparison was downgraded by 2 levels to 'very serious.'
2 There was minimal or no variability in direction of effect, with all (or nearly all) studies favouring the intervention arm. Magnitude of effect varied substantially (4 studies with CIs excluding null). CIs have reasonable overlap. Substantial statistical heterogeneity P = 0.002, I2 = 66%. Therefore, inconsistency was downgraded by one level to 'serious.'
3 Studies retrieved and analysed for this review directly compare the population, interventions and outcomes of interest, as predefined, in our review protocol.
4 Performed optimal information size (OIS) (sample size) calculation, as per GRADE Handbook recommendations, which indicated OIS criterion was met for this outcome. Confidence interval does not include null and includes potential for important benefit.
5 There was minimal or no variability in direction of effect, with all (or nearly all) studies favouring the intervention arm. Magnitude of effect varied substantially (1 study with CI excluding null). Substantial statistical heterogeneity P = 0.0008, I2 = 76%. Therefore, inconsistency was downgraded by one level to 'serious.'
6 Overall risk of bias for this comparison was 'High' for 3/6 and 'some concerns' for the remaining 3/6. In those with high risk, risk derived from missing outcome data (3). The combined weight of the studies with high risk is 44.8%. Therefore, risk of bias for this comparison was downgraded by 2 levels to 'very serious.'
7 There was no variability in direction of effect, with all (or nearly all) studies favouring the intervention arm. Magnitude of effect varied substantially (3 studies with CI excluding null). Substantial statistical heterogeneity P = 0.003, I2 = 70%. Therefore, inconsistency was downgraded by one level to 'serious.'
8 Overall risk of bias for this comparison was 'High' for 5/7 and 'some concerns' for the remaining 2/7. In those with high risk, risk derived from randomisation (1), missing outcome data (3), and selective reporting (1). The combined weight of the studies with high risk is 68.2%.Therefore, risk of bias for this comparison was downgraded by 2 levels to 'very serious.'
Summary of findings 2. Supportive intervention versus control.
| Increased practical support and encouragement during follow‐up + CPAP compared to usual care + CPAP in adults with obstructive sleep apnoea | ||||||
| Patient or population: adults with obstructive sleep apnoea Setting: community Intervention: increased practical support and encouragement during follow‐up + CPAP Comparison: usual care + CPAP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care + CPAP | Risk with Increased practical support and encouragement during follow‐up + CPAP | |||||
| 2.1 CPAP device usage (hours/night) | The mean CPAP device usage ranged from 1.75 to 4.9 hours/night | MD 0.70 hours/night higher (0.36 higher to 1.05 higher) | ‐ | 1426 (13 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 3 4 | |
| 2.2 CPAP device usage, sensitivity analysis: adherence in control group < four hours/night | The mean CPAP device usage, sensitivity analysis: adherence in control group < four hours/night ranged from 1.75 to 3.8 hours/night | MD 0.91 hours/night higher (0.57 higher to 1.25 higher) | ‐ | 735 (7 RCTs) | ⊕⊕⊕⊕ HIGH 3 4 5 | |
| 2.3 N deemed adherent (≥ four hours/night) | 601 per 1,000 | 717 per 1,000 (619 to 797) | OR 1.68 (1.08 to 2.60) | 376 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 6 7 | |
| 2.4 Withdrawals | 136 per 1,000 | 167 per 1,000 (133 to 208) | OR 1.27 (0.97 to 1.66) | 1702 (11 RCTs) | ⊕⊕⊝⊝ LOW 3 8 | |
| 2.5.2 ESS: Comparison Endpoint or Change from Baseline Values ‐ ESS: Change from Baseline | The mean ESS ‐ Comparison Endpoint or Change from Baseline Values ‐ ESS: Change from Baseline ranged from ‐0.7 to ‐5.1 | MD 0.32 lower (1.19 lower to 0.56 higher) | ‐ | 470 (5 RCTs) | ⊕⊕⊝⊝ LOW 3 7 9 | |
| 2.7 Quality of lIfe: Comparison of Change from Baseline Values | The mean Quality of lIfe: Comparison of Change from Baseline Values was 0 | SMD 0.22 higher (0.01 lower to 0.45 higher) | ‐ | 294 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 9 10 11 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CPAP: Continuous positive airways pressure; CI: Confidence interval; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; MD: mean difference; OR: Odds ratio; RCT: randomised controlled trial; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Overall risk of bias for this comparison was 'High' for 8/13 and 'some concerns' for the remaining 5/13. In those with high risk, risk derived from randomisation (1), missing outcome data (6), protocol deviation (1) and selective reporting (2). The combined weight of the studies with high risk is 51.2%. Therefore, risk of bias for this comparison was downgraded by 1 level to 'serious.'
2 Direction of effect had some variability (one study, weight = 6.8%, favoured control), while remaining studies favoured experimental arms. Magnitude of effect varied across studies and CIs had fair overlap. Heterogeneity P = 0.05, I2 = 42%. Heterogeneity explained: attributable to single study with opposite direction of effect (Mendelson 2014). See sensitivity analysis with this study excluded (Analysis 2.13).
3 Studies retrieved and analysed for this review directly compare the population, interventions and outcomes of interest, as predefined, in our review protocol.
4 Performed optimal information size (OIS) (sample size) calculation, as per GRADE Handbook recommendations, which indicated OIS criterion was met for this outcome. Confidence interval does not include null and includes potential for important benefit.
5 Overall risk of bias for this comparison was 'High' for 3/7 and 'some concerns' for the remaining 4/7. In those with high risk, risk derived from missing outcome data (1) and selective reporting (2). The combined weight of the studies with high risk is 14.2%.
6 Overall risk of bias for this comparison was 'High' for 1/2 and 'some concerns' for the remaining 1/2. Hisk risk derived from missing outcome data. The weight of high risk study is 24.8%. Therefore, risk of bias for this comparison was downgraded by 1 level to 'serious.'
7 OIS (sample size) calculation, as per GRADE Handbook recommendations, which indicated OIS criterion not met for this outcome. Therefore, Imprecision for this comparison was downgraded by 1 level to 'serious.'
8 Performed OIS (sample size) calculation, as per GRADE Handbook recommendations, which indicated OIS criterion not met for this outcome. Additionally, CI includes null and potential for important difference in withdrawals. Therefore, Imprecision for this comparison was downgraded by 2 levels to 'very serious.'
9 Overallrisk of bias for this outcome is 'high' for all, or nearly all, included studies because, for all or nearly all studies assessed for this outcome, the following were true: a) outcome assessors (whether participant or investigator) were aware of the intervention received by study participants, b) the outcome assessment could have been influenced by knowledge of the intervention received (because each involves some judgement by the assessor, whether participant or investigator) and c) we have no further information that would permit further adjudication of the likelihood that outcome assessment was influenced by knowledge of the intervention received. Therefore, risk of bias for this comparison was downgraded by 1 level to 'serious.'
10 OIS likely insufficient.Therefore, Imprecision for this comparison was downgraded by 1 level to 'serious.'
11 Our review included a comprehensive search for published reports conducted. All (or nearly all) studies, including all small studies, for this comparison found a benefit for the intervention. Thus, due to suspicion for publication bias, this outcome was downgraded by one level.
12 Overall risk of bias for this comparison was 'High' for 7/12 and 'some concerns' for the remaining 5/12. In those with high risk, risk derived from randomisation (1), missing outcome data (5), protocol deviation (1) and selective reporting (2). The combined weight of the studies with high risk is 46.1%.
Summary of findings 3. Behavioural intervention versus control.
| Behavioural therapy + CPAP compared to control + CPAP in adults with obstructive sleep apnoea | ||||||
| Patient or population: adults with obstructive sleep apnoea Setting: community Intervention: behavioural therapy + CPAP Comparison: control + CPAP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control + CPAP | Risk with Behavioural therapy + CPAP | |||||
| 3.1 CPAP Device Usage (hours/night) | The mean CPAP Device Usage ranged from 1.48 to 5.1 hours/night | MD 1.31 hours/night higher (0.95 higher to 1.66 higher) | ‐ | 578 (8 RCTs) | ⊕⊕⊕⊕ HIGH 1 2 3 | |
| 3.2 CPAP Device Usage, sensitivity analysis: adherence in control group < four hours/night | The mean CPAP Device Usage, sensitivity analysis: adherence in control group < four hours/night ranged from 1.48 to 3.65 hours/night | MD 1.32 hours/night higher (0.93 higher to 1.72 higher) | ‐ | 525 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 4 5 | |
| 3.3 N deemed adherent (≥ four hours/night) | Study population | OR 1.70 (1.20 to 2.41) | 549 (6 RCTs) | ⊕⊕⊕⊕ HIGH 1 6 7 | ||
| 371 per 1,000 | 501 per 1,000 (414 to 587) | |||||
| 3.4 Withdrawal | 146 per 1,000 | 101 per 1,000 (70 to 143) | OR 0.66 (0.44 to 0.98) | 939 (10 RCTs) | ⊕⊕⊕⊕ HIGH | |
| 3.5 ESS (Endpoint scores) | The mean ESS (Endpoint scores) ranged from 7.1 to 12.5 | MD 2.42 lower (4.27 lower to 0.57 lower) | ‐ | 271 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 8 9 | |
| 3.6 AHI on treatment ‐ Endpoint | The mean AHI at endpoint ranged from 3.7 to 4.3 events/hour | MD 0.95 events/hour lower (2.25 lower to 0.34 higher) | ‐ | 89 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 10 11 | |
| 3.7 Quality of Life ‐ Comparison of Values at Endpoint | The mean Quality of Life ‐ Comparison of Values at Endpoint was 0 | SMD 0 (0.26 lower to 0.26 higher) | ‐ | 228 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 8 | |
| 3.7.1 Quality of Life ‐ Comparison of Values at Endpoint ‐ QoL: FOSQ ‐ Endpoint | The mean Quality of Life ‐ Comparison of Values at Endpoint ‐ QoL: FOSQ ‐ Endpoint was 0 | SMD 0.01 higher (0.26 lower to 0.29 higher) | ‐ | 200 (2 RCTs) | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; CI: Confidence interval; CPAP: Continuous positive airways pressure;ESS: Epworth sleepiness scale; FOSQ: Functional Outcomes of Sleep Questionnaire; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; MD: mean difference; OR: Odds ratio; RCT: randomised controlled trial; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Studies retrieved and analysed for this review directly compare the population, interventions and outcomes of interest, as predefined, in our review protocol.
2 Performed optimal information size (OIS) (sample size) calculation, as per GRADE Handbook recommendations, which indicated OIS criterion was met for this outcome. Confidence interval does not include null and includes potential for important benefit (1 hour more use/night).
3 Overall risk of bias for this comparison was 'Some concerns' for 4/8 and 'high' for the remaining 4/8. In those with high risk, risk derived from randomisation (1), missing outcome (1), protocol deviation/missing outcome data (1) and selective reporting (1). The combined weight of the four studies with high risk is 45.1%.
4 Overall risk of bias for this comparison was 'Some concerns' for 3/6 and 'high' for the remaining 3/6. In those with high risk, risk derived from missing outcome (1), protocol deviation/missing outcome data (1) and selective reporting (1). The combined weight of the two studies with high risk is 54.4%. Therefore, risk of bias for this comparison was downgraded by 1 level to 'serious.'
5 Direction of effect did not vary. Magnitude of effect varied somewhat and CIs had good overlap. Heterogeneity P = 0.38, I2 = 6%.
6 Overall risk of bias for this comparison was 'Some concerns' for 2/6 and 'high' for the remaining 4/6. In those with high risk, risk derived from randomisation process (1), missing outcome data (1), protocol deviation/missing outcome data (1) and selective reporting (1). The combined weight of the two studies with high risk is 32.4%.
7 One (second highest‐weighted) study found opposite direction of effect (favoured control). The remaining studies had similar magnitude of effect and showed reasonable overlap of CIs. Heterogeneity P = 0.46, I2 = 0%.
8 Overall risk of bias for this outcome is 'high' for all, or nearly all, included studies because, for all or nearly all studies assessed for this outcome, the following were true: a) outcome assessors (whether participant or investigator) were aware of the intervention received by study participants, b) the outcome assessment could have been influenced by knowledge of the intervention received (because each involves some judgement by the assessor, whether participant or investigator) and c) we have no further information that would permit further adjudication of the likelihood that outcome assessment was influenced by knowledge of the intervention received. Therefore, risk of bias for this comparison was downgraded by 1 level to 'serious.'
9 Direction of effect had some variability (one study, weight =17.9%, modestly favoured control), while remaining studies favoured experimental arms. Magnitude of effect varied significantly and CIs had moderate overlap. Heterogeneity P = 0.008, I2=71%.Therefore, inconsistency was downgraded by one level to 'serious.'
10 Only two studies provided information for this comparison. Overall risk of bias for this comparison was 'Some concerns' for 1/2 and 'high' for the remaining 1/2 (Diaferia 2017). High‐risk derived from protocol deviation/missing outcome data. Additionally, the other study (Dantas 2015) had 'some concerns' for domain 1 (study level), randomisation process. Therefore, risk of bias for this comparison was downgraded by 1 level to 'serious.'
11 Performed OIS (sample size) calculation, as per GRADE Handbook recommendations, which indicated OIS criterion not met for this outcome. Additionally, CI contained null effect and potential for important benefit.Therefore, Imprecision for this comparison was downgraded by 2 levels to 'very serious.'
Summary of findings 4. Mixed (BEH/EDU/SUP) intervention versus control.
| Mixed (SUP/EDU/BEH) Intervention + CPAP compared to Usual Care + CPAP in adults with obstructive sleep apnoea | ||||||
| Patient or population: adults with obstructive sleep apnoea Setting: community Intervention: mixed (SUP/EDU/BEH) Intervention + CPAP Comparison: usual Care + CPAP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Usual Care + CPAP | Risk with Mixed (SUP/EDU/BEH) Intervention + CPAP | |||||
| 4.1 CPAP Device Usage (hours/night) | The mean CPAP Device Usage ranged from 2.6 to 5.5 hours/night | MD 0.82 hours/night higher (0.20 higher to 1.43 higher) | ‐ | 4509 (11 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | |
| 4.2 CPAP Device Usage, sensitivity analysis: adherence in control group < four hours/night | The mean CPAP Device Usage, sensitivity analysis: adherence in control group < four hours/night ranged from 2.6 to 3.8 hours/night | MD 1.77 hours/night higher (0.21 higher to 3.34 higher) | ‐ | 343 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 5 6 | |
| 4.3 N deemed adherent (≥ four hours/night) | 741 per 1,000 | 830 per 1,000 (755 to 886) | OR 1.71 (1.08 to 2.72) | 4015 (9 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 7 8 | |
| 4.4 Withdrawal | 129 per 1,000 | 83 per 1,000 (40 to 161) | OR 0.61 (0.28 to 1.30) | 4956 (11 RCTs) | ⊕⊝⊝⊝ VERY LOW 9 10 11 | |
| 4.5 Quality of LIfe: Comparison of Change from Baseline Values | The mean Quality of LIfe: Comparison of Change from Baseline Values was 0 | SMD 0.45 higher (0.12 higher to 0.78 higher) | ‐ | 3012 (2 RCTs) | ⊕⊕⊝⊝ LOW 12 13 14 | |
| 4.7 Anxiety Symptom Rating ‐ Comparison of Values at Endpoint | The mean Anxiety Symptom Rating ‐ Comparison of Values at Endpoint was 0 | SMD 0.19 lower (0.47 lower to 0.09 higher) | ‐ | 333 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 12 15 16 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CPAP: Continuous positive airways pressure; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; MD: mean difference; OR: Odds ratio; RCT: randomised controlled trial; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Overall risk of bias for this comparison was 'Some concerns' for 4/11 and 'high' for the remaining 6/11. In those with high risk, risk derived from randomisation (2), missing outcome data (2), and selective reporting (3). The combined weight of the studies with high risk is 61.8%. (1 high risk study. Lewis 2006, has no weight contribution because mean difference not estimable.) Therefore, risk of bias for this comparison was downgraded by 1 level to 'serious.'
2 Direction of effect had some variability (two studies, combined weight =18.8%, favoured control), while remaining studies favoured experimental arms. Magnitude of effect varied significantly and CIs had relatively poor overlap. Heterogeneity P < 0.00001, I2 = 92% suggesting very substantial statistical heterogeneity of effect. Therefore, inconsistency was downgraded by two levels to 'very serious.'
3 Studies retrieved and analysed for this review directly compare the population, interventions and outcomes of interest, as predefined, in our review protocol.
4 Performed optimal information size (OIS) (sample size) calculation, as per GRADE Handbook recommendations, which indicated OIS criterion was met for this outcome. Confidence interval does not include null and includes potential for important benefit.
5 Overall risk of bias for this comparison was 'Some concerns' for 1/2 and 'high' for the remaining 1/2. In those with high risk, risk derived from missing outcome data. The weight of the high risk study is 48.3%.Because there were only two studies for this comparison and both were either high or 'some concerns,' risk of bias for this comparison was downgraded by 1 level to 'serious.'
6 There was no variability in direction of effect, both studies favoured experimental arms. Magnitude of effect varied substantially and CIs had no overlap. Heterogeneity P = 0.002, I2 = 90% suggesting very substantial statistical heterogeneity of effect. Therefore, inconsistency was downgraded by two levels to 'very serious.'
7 Overall risk of bias for this comparison was 'high' for 4/9. In those with high risk, risk derived from randomisation (1), missing outcome data (1), and selective reporting (2). The combined weight of the studies with high risk is 51.3%. Therefore, risk of bias for this comparison was downgraded by 1 level to 'serious.'
8 There was variability in direction of effect (three studies, combined weight=31,6%, favoured control), while remaining studies favoured experimental arms. Magnitude of effect varied substantially and CIs had modest overlap. Heterogeneity P < 0.00001, I2 = 79% suggesting very substantial statistical heterogeneity of effect.Therefore, inconsistency was downgraded by two levels to 'very serious.'
9 Performed OIS (sample size) calculation, as per GRADE Handbook recommendations, which indicated OIS criterion was met for this outcome. Confidence interval includes null and includes potential for important benefit.Therefore, imprecision was downgraded by 1 level to 'serious.'
10 There was variability in direction of effect (five studies, combined weight = 35.4%, favoured control), while remaining studies favoured experimental arms. Magnitude of effect varied substantially and CIs had modest overlap. Heterogeneity P < 0.00001, I2 = 85% suggesting very substantial statistical heterogeneity of effect.Therefore, inconsistency was downgraded by two levels to 'very serious.'
11 Overall risk of bias for this comparison was 'high' for 6/11 studies. In those with high risk, risk derived from randomisation (2), missing outcome data (2), and selective reporting (2). The combined weight of the studies with high risk is 52.80%. Therefore, risk of bias for this comparison was downgraded by 1 level to 'serious.'
12 Overall risk of bias for this outcome is 'high' for all, or nearly all, included studies because, for all or nearly all studies assessed for this outcome, the following were true: a) outcome assessors (whether participant or investigator) were aware of the intervention received by study participants, b) the outcome assessment could have been influenced by knowledge of the intervention received (because each involves some judgement by the assessor, whether participant or investigator) and c) we have no further information that would permit further adjudication of the likelihood that outcome assessment was influenced by knowledge of the intervention received.Therefore, risk of bias for this comparison was downgraded by 1 level to 'serious.'
13 There was no variability in direction of effect, both studies favoured experimental arms. Magnitude of effect varied substantially and CIs had minimal overlap. Heterogeneity P = 0.03, I2 = 79% suggesting considerable heterogeneity of effect.Therefore, inconsistency was downgraded by 1 level to 'serious.'
14 Sample size likely sufficient. Confidence interval does not include null, but also likely does not include potential for important benefit (i.e. standardised mean difference of at least 1). No downgrade.
15 Overall risk of bias for this outcome is 'high' for all, or nearly all, included studies because, for all or nearly all studies assessed for this outcome, the following were true: a) outcome assessors (whether participant or investigator) were aware of the intervention received by study participants, b) the outcome assessment could have been influenced by knowledge of the intervention received (because each involves some judgement by the assessor, whether participant or investigator) and c) we have no further information that would permit further adjudication of the likelihood that outcome assessment was influenced by knowledge of the intervention received. Additionally, a different anxiety symptom rating scale was used for each and they targeted different dimensions of anxiety (e.g. state vs. trait). Therefore, risk of bias for this comparison was downgraded by 2 levels to 'very serious.'
16 Sample size for this comparison relatively small, OIS probably not met (approximated based on comparison of means for study with highest weight). CI includes null but likely does not include important benefit/harm.Therefore, imprecision was downgraded by 1 level to 'serious.'
Please refer to the 'Summary of findings' tables for each comparison group.
Table 1 Educational support for adults with obstructive sleep apnoea who are using CPAP
Table 2 Increased support and encouragement for adults with obstructive sleep apnoea
Table 3 Behavioral therapy and encouragement for adults with obstructive sleep apnoea
Table 4 Mixed interventions for adults with obstructive sleep apnoea
Several post hoc sensitivity analyses were also conducted (Table 12; Table 13; Table 14; Table 15; Table 16), and are described in Quality of the evidence .
8. Post‐hoc sensitivity analysis: effect of high 'Risk of bias' studies.
| Class | Full class effect estimate, MD (95% CI) | Sensitivity: excluding high RoB studies (MD, 95%CI) |
| Behavioural | 1.31 (0.95 to 1.66) I2 = 0% Analysis 3.1 |
1.05 (0.57 to 1.53)1 I2 = 0% Analysis 5.6 |
| Educational | 0.85 (0.32 to 1.39) I2 = 68% Analysis 1.1 |
0.98 (0.07 to 1.89)2 I2 = 86% Analysis 5.4 |
| Supportive | 0.70 (0.36 to 1.05) I2 = 42% (Analysis 2.1) |
0.75 (0.42 to 1.09)3 I2 = 34% Analysis 5.5 |
| Mixed | 0.82 (0.20 to 1.43) I2 = 92% Analysis 4.1 |
NA |
1. Included in sensitivity analysis: Aloia 2013; Bakker 2016; Dantas 2015; Olsen 2012
2. Included in sensitivity analysis: Aloia 2013; Basoglu 2011; Hwang 2017; Richards 2007
3. Included in sensitivity analysis: Fox 2012; Hoy 1999; Hwang 2017; Stepnowsky 2013; Turino 2017
9. Post‐hoc subgroup analysis: effects of intervention duration, contact episodes, contact time.
| Class | Full class effect estimate, MD (95%CI) | Intervention duration, MD (95%CI) | Contact episodes: 1 vs. > 1, MD (95%CI) | Total contact time: > vs. ≤ 60 minutes, MD (95%CI) |
| Behavioral | 1.31 (0.95 to1.66) I2 = 0% Analysis 3.1 |
> 4 weeks1: 1.21 (0.60 to 1.82) I2 = 0% ≤ 4 weeks2: 1.38 (0.80 to 1.95) I2 = 38% Test for subgroup differences: Chi² = 0.15, df = 1 (P = 0.70), I² = 0% Analysis 6.7 |
> 1 episode3: 1.35 (0.94 to 1.77) I2 = 9% 1 episode4: 1.10 (0.26 to1.94) I2 = 0% Test for subgroup differences: Chi² = 0.28, df = 1 (P = 0.60), I² = 0% Analysis 6.8 |
> 60 minutes5: 1.15 (0.71 to 1.60); I2 = 0% ≤ 60 minutes6: 1.56 (0.68 to 2.44); I2 = 57% Test for subgroup differences: Chi² = 0.64, df = 1 (P = 0.42), I² = 0% Analysis 6.9 |
| Educational | 0.85 (0.32 to 1.39) I2 = 68% Analysis 1.1 |
> 4 weeks7: 0.33 (‐0.10 to 0.77); I2 = 0% ≤ 4 weeks8: 1.20 (0.39 to2.01); 12=75% Test for subgroup differences: Chi² = 3.36, df = 1 (P = 0.07), I² = 70.2% Analysis 6.1 |
> 1 episode9: 1.20 (0.41 to2.00); I2 = 70% 1 episode10: 0.40 (‐0.06 to 0.86); I2 = 0% Test for subgroup differences: Chi² = 2.96, df = 1 (P = 0.09), I² = 66.2% Analysis 6.2 |
> 60 minutes11: 1.46 (0.22 to 2.71); I2 = 82% ≤ 60 minutes12: 0.61 (0.00 to 1.22); I2 = 37% Test for subgroup differences: Chi² = 1.47, df = 1 (P = 0.23), I² = 31.9% Analysis 6.3 |
| Supportive | 0.70 (0.36 to 1.05) I2 = 42% (Analysis 2.1) |
> 12 weeks13: 0.49 (‐0.53 to 1.51); I2 = 77% ≤ 12 weeks14: 0.72 (0.43 to 1.01); I2 = 0% Test for subgroup differences: Chi² = 0.17, df = 1 (P = 0.68), I² = 0% Analysis 6.4 |
NA | NA |
| Mixed | 0.82 (0.20 to 1.43) I2 = 92% Analysis 4.1 |
> 4 weeks15: 1.22 (0.60 to 1.83); I2 = 91% ≤ 4 weeks16: ‐0.31 (‐0.83 to 0.21); I2 = 0% Test for subgroup differences: Chi² = 13.79, df = 1 (P = 0.0002), I² = 92.7% Analysis 6.10 |
> 1 episode17: 0.98 (0.32 to 1.62); I2 = 92% 1 episode18: ‐0.60 (‐1.33 to 0.13); I2 = 93% Test for subgroup differences: Chi² = 9.94, df = 1 (P = 0.002), I² = 89.9% Analysis 6.11 |
> 60 minutes19: 1.45 (0.73 to 2.16); I2 = 91% ≤ 60 minutes20: ‐0.15 (‐0.56 to 0.27); I2 = 0% Test for subgroup differences: Chi² = 14.14, df = 1 (P = 0.0002), I² = 92.9% Analysis 6.12 |
1. Bakker 2016; Diaferia 2017; Wang 2012
2. Aloia 2001; Aloia 2013; Dantas 2015; Lai 2014; Olsen 2012
3. Aloia 2001; Aloia 2013; Bakker 2016; Diaferia 2017; Lai 2014; Olsen 2012; Wang 2012
4. Dantas 2015
5. Aloia 2001; Aloia 2013; Bakker 2016; Diaferia 2017; Olsen 2012; Wang 2012
6. Dantas 2015; Lai 2014
7. Hwang 2017; Pengo 2018; Wang 2012
8. Aloia 2013; Basoglu 2011; Chervin 1997; Falcone 2014; Richards 2007; Roecklein 2010; Sarac 2017
9. Aloia 2013; Chervin 1997; Richards 2007; Sarac 2017; Pengo 2018; Wang 2012
10. Basoglu 2011; Falcone 2014; Roecklein 2010
11. Aloia 2013; Richards 2007; Wang 2012
12. Basoglu 2011; Chervin 1997; Falcone 2014; Sarac 2017
13. Hoy 1999; Mendelson 2014; Parthasarathy 2013; Pepin 2019
14. Chervin 1997; DeMolles 2004; Fox 2012; Hoet 2017; Hwang 2017; Munafo 2016; Stepnowsky 2007; Stepnowsky 2013; Turino 2017
15. Bouloukaki 2014; Chen 2015; Hui 2000; Hwang 2017; Meurice 2007; Sedkaoui 2015; Wang 2012
16. Bartlett 2013; Sawyer 2017; Shapiro 2017
17. Bouloukaki 2014; Chen 2015; Hui 2000; Meurice 2007; Sawyer 2017; Sedkaoui 2015; Shapiro 2017; Wang 2012
18. Bartlett 2013
19. Bouloukaki 2014; Chen 2015; Sawyer 2017; Sedkaoui 2015; Wang 2012
10. Post‐hoc subgroup analysis: effect of human support components in supportive interventions.
| Class | All supportive interventions, MD (95%CI) | Intervention involved human support, MD (95%CI) | Intervention involved scheduled human support, MD (95%CI) |
| Supportive | 0.70 (0.35 to 1.05) I2 = 42% (Analysis 2.1) |
Any human support1: 0.84 (0.52 to 1.17) I2 = 10% Automated support only2: 0.26 (‐0.51 to 1.04) I2 = 64% Test for subgroup differences: Chi² = 1.85, df = 1 (P = 0.17), I² = 46.0% Analysis 6.5 |
Pre‐scheduled human support3: 1.43 (0.61 to 2.24) I2 = 0% No Scheduled human support4: 0.58 (0.33 to 0.83) I2 = 45% Test for subgroup differences: Chi² = 3.82, df = 1 (P = 0.05), I² = 73.8% Analysis 6.6 |
1. Chervin 1997; Fox 2012; Hoet 2017; Hoy 1999; Parthasarathy 2013; Pepin 2019; Stepnowsky 2007; Stepnowsky 2013; Turino 2017
2. DeMolles 2004; Hwang 2017; Mendelson 2014; Munafo 2016
3. Chervin 1997; Hoy 1999; Parthasarathy 2013
4. DeMolles 2004; Fox 2012; Hoet 2017; Hwang 2017; Mendelson 2014; Munafo 2016; Pepin 2019; Stepnowsky 2007; Stepnowsky 2013; Turino 2017
11. Post‐hoc sensitivity analysis: effect of intervention classification decisions.
| Class | Updated Review (Askland 2019) Classification Decision, MD (95%CI) | Sensitivity: Original (Wozniak 2014) Classification Decision, MD (95%CI) |
| Behavioral | 1.31 (0.95 to1.66) I2 = 0% Analysis 3.1 |
1.47 (1.12 to 1.83) I2 = 48% Analysis 5.3 |
| Educational | 0.85 (0.32 to 1.39) I2 = 68% Analysis 1.1 |
0.48 (0.21 to 0.76) I2 = 0% Analysis 5.1 |
| Supportive | 0.70 (0.36 to 1.05) I2 = 42% (Analysis 2.1) |
0.58 (0.36 to 0.81) I2 = 45% Analysis 5.2 |
12. Post‐hoc sensitivity analysis: effect of selection of (closest to) 3‐month endpoint.
| Class | Full Class Effect Estimates | Exclude endpoints NOT 3 months |
| Behavioral | 1.31 (0.95 to 1.66) I2 = 0% Analysis 3.1 |
1.38 (0.97 to 1.79) |
| Educational | 0.85 (0.32 to 1.39) I2 = 68% Analysis 1.1 |
0.63 (0.26 to 1.00) |
| Supportive | 0.70 (0.36 to 1.05) I2 = 42% (Analysis 2.1) |
0.67 (0.29 to 1.04) |
| Mixed | 0.82 (0.20 to 1.43) I2 = 92% Analysis 4.1 |
1.09 (0.21 to 1.97) |
Full class effect estimates are those derived in our primary analyses, which includes the data from each included study closest to our primary 3‐month endpoint. That is, if no 3‐month endpoint data were available for a study, the endpoint closest to (and later than) 3 months was used. For example if a study reported data at 2 months and 4 months post‐intervention, the 4‐month endpoint data were used. If only a single endpoint was reported by authors (e.g. Bouloukaki 2014 reported only 2‐year endpoint), data for that endpoint was used.
Primary outcome: CPAP usage
Mean hours/night
Educational interventions
Very low‐certainty evidence showed that educational interventions increased average hours of CPAP use (mean difference (MD) 0.85, 95% confidence interval (CI) 0.32 to 1.39; participants = 1128; studies = 10; I2 = 68%), Analysis 1.1; Figure 3). Substantial statistical heterogeneity was detected (I2 = 68%, P = 0.002) due to poor overlap of estimates and CIs, most notably, the increased effects presented by two studies (Falcone 2014; Richards 2007), leading to a downgrading of certainty by one level. Moreover, the standard deviations (SDs) reported by Falcone 2014 were considerably smaller than the other studies in this comparison (and the reported SDs were inconsistent with reported P values for the comparison), suggesting the possibility of spurious confidence in the estimate. Therefore, Falcone 2014 SDs were therefore entered as zero values, so the Falcone 2014 data did not contribute to overall meta‐analysis and does not appear in the forest plot. However heterogeneity remained significant (resulting in downgrading in this domain). Further downgrading by two levels was also applied in the 'Risk of bias' domain, as the combined weight of studies with "high" risk was 70%, and was due to bias in randomisation procedures (Sarac 2017), missing outcome data (Chervin 1997; Falcone 2014; Pengo 2018; Roecklein 2010; Wang 2012), deviations from intended interventions (Chervin 1997), and selective reporting (Basoglu 2011). Therefore, the overall certainty of the evidence for this outcome was downgraded by three levels, yielding a 'very‐low' rating.
1.1. Analysis.
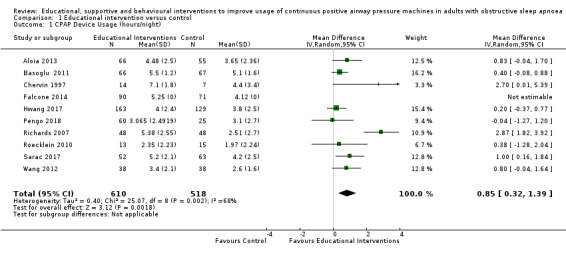
Comparison 1 Educational intervention versus control, Outcome 1 CPAP Device Usage (hours/night).
3.
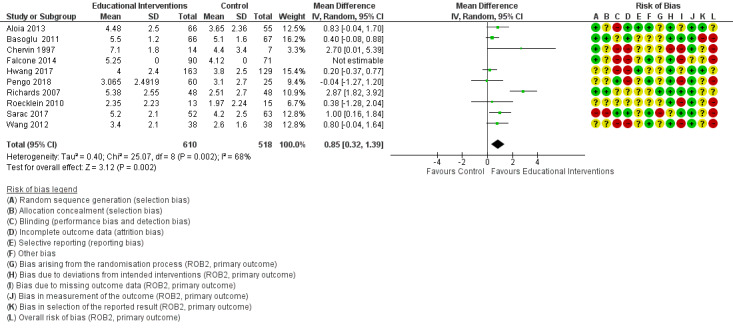
Forest plot of comparison: 1 Educational intervention versus control on primary outcome: CPAP Device Usage (hours/night).
A sensitivity analysis was performed, where studies with average usage in the control group > 4 hours/night were excluded (Basoglu 2011; Chervin 1997; Falcone 2014; Sarac 2017). Very low‐certainty evidence demonstrated that educational interventions increased usage (MD 0.85, 95% CI 0.06 to 1.64), based on 6 studies with 698 participants. Heterogeneity in this sensitivity analysis remained high (I2 = 76%), due to the inclusion of Richards 2007's larger effect estimate, leading to poor overlap of CIs. Certainty of evidence was further downgraded by two levels as the combined weight of studies high risk of bias (3/6 studies) was 44.8%, with the remaining studies having some risk concerns.Therefore, overall certainty for this outcome was reduced by three levels, yielding a 'very low‐certainty' rating.
Supportive interventions
Moderate‐certainty evidence showed that supportive interventions increased average hours of CPAP use (MD 0.70, 95% CI 0.36 to 1.05; participants = 1426; studies = 13; I2 = 42%) (Analysis 2.1; Figure 4). Moderate heterogeneity (I2 = 42%, P = 0.05) was accounted for by the opposite direction of effect observed in Mendelson 2014, where the mean difference favoured the control group. In studies that had "high" overall risk of bias, risk derived from randomisation procedures (Hoet 2017), missing outcome data (Chervin 1997; Hoet 2017; Mendelson 2014; Munafo 2016; Pepin 2019; Stepnowsky 2007), protocol deviation (Chervin 1997), selective outcome reporting (DeMolles 2004; Parthasarathy 2013), and had a combined analysis weight of 51.2%, warranting downgrading by one level in this domain. Therefore, the overall certainty of the evidence was downgraded by one level, yielding a 'moderate' rating.
2.1. Analysis.
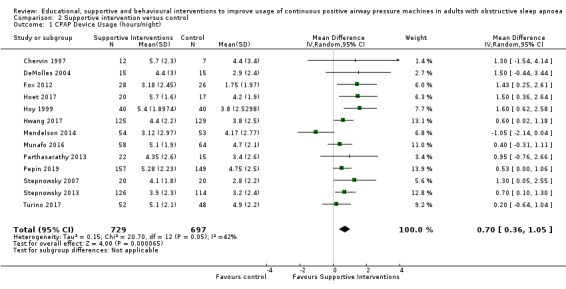
Comparison 2 Supportive intervention versus control, Outcome 1 CPAP Device Usage (hours/night).
4.
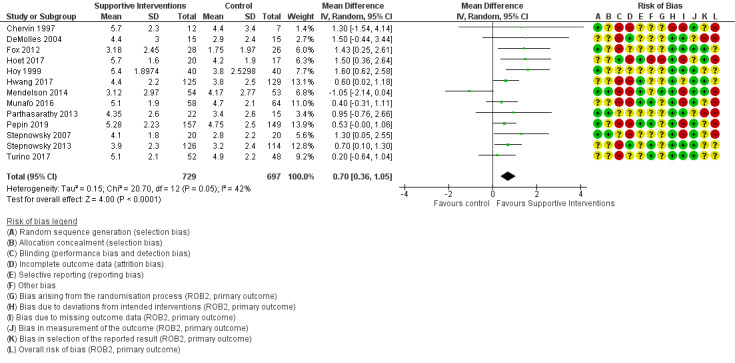
Forest plot of comparison: 2 Supportive intervention versus control on primary outcome: CPAP Device Usage (hours/night).
A sensitivity analysis Analysis 2.2 was conducted to determine the influence of studies that had an average usage > 4 hours per night in the control group. High‐certainty evidence demonstrated that supportive intervention increased average hours of CPAP use (MD 0.91, 95% CI 0.57 to 1.25; participants = 735; studies = 7; I2 = 0%).
2.2. Analysis.
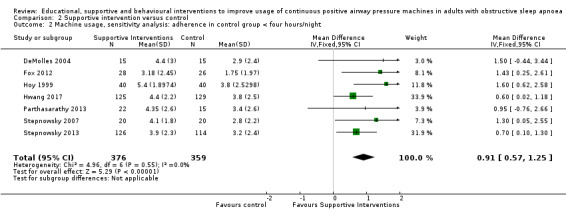
Comparison 2 Supportive intervention versus control, Outcome 2 Machine usage, sensitivity analysis: adherence in control group < four hours/night.
An additional post‐hoc sensitivity analysis was conducted (Analysis 2.9), excluding the single study with opposite direction of effect (Mendelson 2014). In their conclusions, trialists indicated that their findings in favour of the control arm may have derived specifically from the "additional burden associated with the self‐management of BP and CPAP by patients randomised to this group," a study component and outcome not germane to our review. Exclusion of this study resulted in a slight increase in the effect estimate (and narrower CIs) as well as the elimination of heterogeneity (MD 0.74, 95% CI 0.49 to 0.98; participants = 1319; studies = 12; I2 = 0%).
2.9. Analysis.
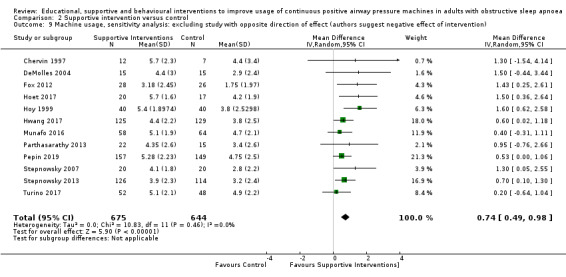
Comparison 2 Supportive intervention versus control, Outcome 9 Machine usage, sensitivity analysis: excluding study with opposite direction of effect (authors suggest negative effect of intervention).
Behavioural interventions
High‐certainty evidence showed that behavioural interventions increased average hours of CPAP use (MD 1.31, 95% CI 0.95 to 1.66; participants = 578; studies = 8; I2 = 0%) (Analysis 3.1; Figure 5). No heterogeneity was detected for this outcome indicating that magnitude and direction of effect estimates were similar. Certainty of this effect estimate for this outcome (as per GRADE assessment procedures) was judged to be high.
5.
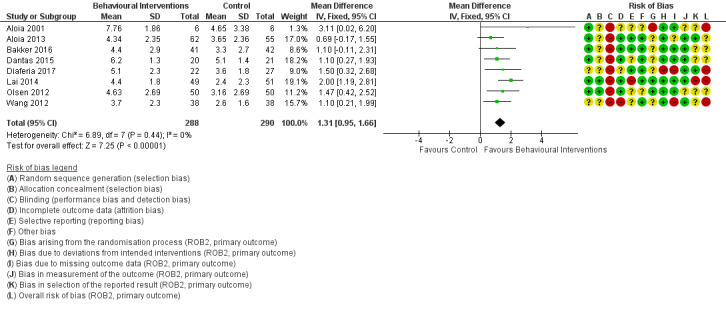
Forest plot of comparison: 3 Behavioural intervention versus control on primary outcome: CPAP Device Usage (hours/night).
A pre‐defined sensitivity analysis Analysis 3.2 was conducted to exclude studies where average CPAP adherence in the control group was more than four hours per night (excluded Aloia 2001; Dantas 2015). Moderate‐certainty evidence showed that behavioural interventions increased average hours of CPAP use (MD 1.32, 95% CI 0.93 to 1.72; participants = 525; studies = 6; I2 = 6%). Confidence in this effect estimate was downgraded by one level due to high risk of bias in studies accounting for 54.4% of estimate weight.
3.2. Analysis.
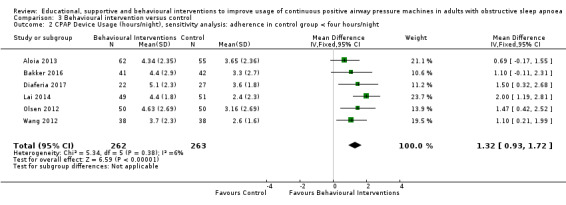
Comparison 3 Behavioural intervention versus control, Outcome 2 CPAP Device Usage (hours/night), sensitivity analysis: adherence in control group < four hours/night.
Mixed interventions
Very low‐certainty evidence showed that mixed interventions increased average hours of CPAP used (MD 0.82, 95% CI 0.20 to 1.43; participants = 4509; studies = 11; I2 = 92%) (Analysis 4.1; Figure 6). Substantial statistical heterogeneity was due to variability in direction of effect (Bartlett 2013 and Shapiro 2017 favoured the control group), variability in magnitude of effects, and poor overlap of CIs, warranting a downgrading of certainty by two levels. Studies in this comparison presented as having a "high" risk of bias, deriving from risk in randomisation procedures (Hui 2000; Meurice 2007), missing outcome data (Wang 2012), and selective reporting (Chen 2015; Sedkaoui 2015). The combined weight of these studies was 50.2%, warranting further downgrading of certainty in this domain.Therefore, the overall certainty of the evidence for this outcome was downgraded by three levels, yielding a 'very‐low ' rating.
4.1. Analysis.
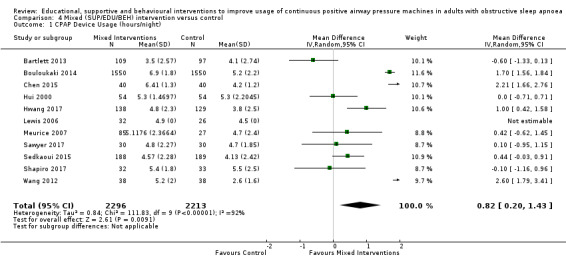
Comparison 4 Mixed (SUP/EDU/BEH) intervention versus control, Outcome 1 CPAP Device Usage (hours/night).
6.
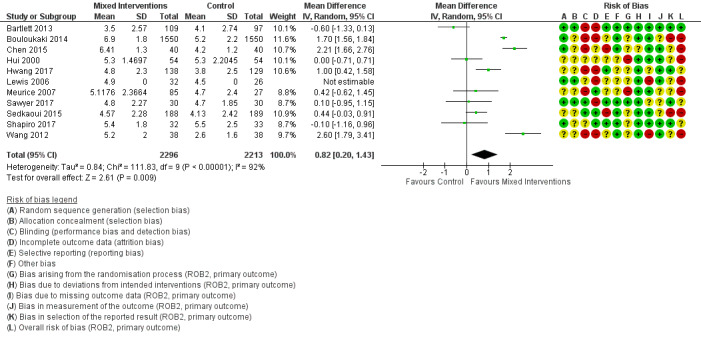
Forest plot of comparison: 4 Mixed (SUP/EDU/BEH) intervention versus control on primary outcome: CPAP Device Usage (hours/night).
When excluding studies where average usage was greater than four hours per night in the control group (Bartlett 2013; Bouloukaki 2014; Chen 2015; Hui 2000; Meurice 2007; Sawyer 2017; Sedkaoui 2015; Shapiro 2017), the two remaining mixed interventions (Hwang 2017; Wang 2012) increased hours of use (MD 1.77, 95% CI 0.21 to 3.34; participants = 343; studies = 2; I2 = 90%), however this evidence was also very low in certainty due high heterogeneity (I2 = 90%, P = 0.002), warranting downgrading by two levels, as well as high risk associated with missing outcome data (Wang 2012, study weight = 48.3%), warranting downgrading by a further level.
Secondary outcomes
Data were not available for all of the secondary outcomes specified in the protocol. Those with available data are described below. For some secondary outcomes, some studies reported change‐from‐baseline measurements, whereas other studies reported endpoint values only. In these cases, values were combined in a single meta‐analysis using MD as the effect measure. In cases where a study reported both change‐from‐baseline with SD and endpoint values for a given outcome, only change‐from‐baseline data were used in the meta‐analysis. If an outcome was evaluated using different measures across studies, and, therefore, required use of standardised mean difference (SMD) as the effect measure, separate meta‐analyses were performed for studies reporting change‐from‐baseline and for those reporting only endpoint comparisons.
Number of participants deemed adherent (average CPAP usage ≥ 4 hours/night)
Very low‐certainty evidence from 7 studies and 1019 participants (Table 1; Analysis 1.3) showed that educational interventions increased the number of people deemed adherent when compared to control (odds ratio (OR) 2.58, 95% CI 1.50 to 4.44, P = 0.003), translating to an absolute risk increase from 558 to 765 (95% CI: 654 to 849) people per 1000. Certainty of evidence in this comparison was downgraded by two levels due to high risk of bias in seven studies (Basoglu 2011; Falcone 2014; Sarac 2017; Soares‐Pires 2013; Wang 2012), deriving from randomisation procedures, missing outcome data and selective outcome reporting with a combined analysis weight of 68.1%. Further downgrading by one level was performed due detection of considerable statistical heterogeneity (I2= 76%). Therefore, the overall certainty of the evidence for this outcome was downgraded by three levels, yielding a 'very low ' rating.
1.3. Analysis.
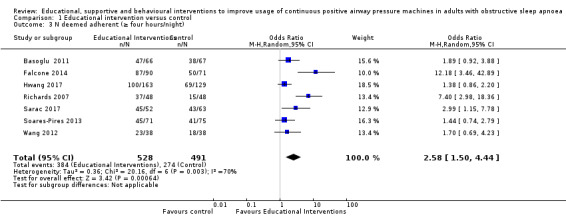
Comparison 1 Educational intervention versus control, Outcome 3 N deemed adherent (≥ four hours/night).
Low‐certainty evidence from 2 studies and 376 participants (Table 2; Analysis 2.3) showed that supportive interventions increased the number of people deemed adherent when compared to control (OR 1.68, 95% CI 1.08 to 2.60, P = 0.02), translating to an absolute risk increased from 601 to 717 (95% CI: 619 to 797) people per 1000. Evidence in this comparison was downgraded by one level due to the low sample size, as defined by GRADE's recommendations on optimal information size (OIS) (Schunemann 2013). Evidence was further downgraded by one level due to high risk of bias from one study (24.8% weight) and some risk concerns in the other study. Therefore, the overall certainty of the evidence was downgraded by two levels, yielding a 'low ' rating.
2.3. Analysis.
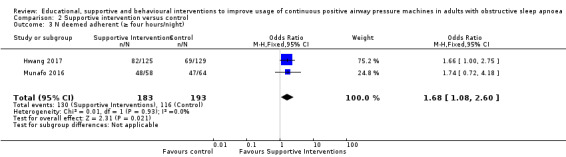
Comparison 2 Supportive intervention versus control, Outcome 3 N deemed adherent (≥ four hours/night).
High‐certainty evidence from 6 studies and 549 participants (Table 3; Analysis 3.3) indicated that behavioural interventions increased the number of people deemed adherent (based on author‐defined nightly CPAP use threshold) when compared to control (OR 1.70, 95% CI 1.20 to 2.41, P = 0.003). Based on average control group risk, this translates to an absolute risk increase from 371 to 501 (95% CI: 414 to 587) people per 1000.
3.3. Analysis.
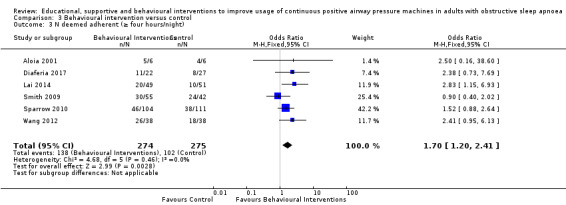
Comparison 3 Behavioural intervention versus control, Outcome 3 N deemed adherent (≥ four hours/night).
Very low‐certainty evidence from 9 studies and 4015 participants (Table 4; Analysis 4.3) indicated that mixed interventions increased the number of people deemed adherent when compared to control (OR 1.71, 95% CI 1.08 to 2.72, P < 0.001), translating to an absolute risk increased from 741 to 830 (95% CI: 755‐886) people per 1000. Evidence in this comparison was downgraded by one level as the combined weight of studies with high risk was 51.3%, and was downgraded another two levels level due to substantial heterogeneity (I2 = 79%, P < 0.001). Therefore, the overall certainty of the evidence was downgraded by three levels, yielding a 'very‐low' rating.
4.3. Analysis.
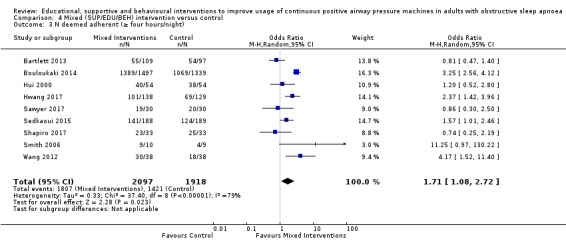
Comparison 4 Mixed (SUP/EDU/BEH) intervention versus control, Outcome 3 N deemed adherent (≥ four hours/night).
Withdrawals
One of our pre‐specified secondary outcomes was "study withdrawals." Generally, the primary objective in reporting study withdrawals, and conducting meta‐analysis on this outcome, is to assess the potential impact of attrition bias on reported effect estimates. Studies with lower attrition (and attrition bias) should produce more valid effect estimates. Across included studies in our review, trialists varied substantially in: a) whether study withdrawal was assessed/reported as an outcome, b) if "withdrawal" was explicitly defined (and the term applied by trialists – e.g. "drop‐outs," "withdrawal," "lost‐to‐follow‐up," "unable to make contact," etc....), and c) among those providing an explicit definition, how withdrawal (or other comparable term) was defined. Due to this substantial variability, we concluded that no single author‐employed term or definition would have permitted consistent accounting across studies. We therefore decided to employ a definition that permitted a common, objective measure that is not dependent on author definition and is less dependent on author reporting decisions: we counted as a withdrawal any participant who, subsequent to randomisation withdrew from the study, such that their actual CPAP device usage could not be determined. Participants who withdrew from participation in the intervention only (i.e. a) returned their CPAP device prior to the start of the intervention or at any subsequent point, or b) whose CPAP device usage was transmitted wirelessly; in both cases, device data for the period of the intervention were accessible to trialists for analysis) were not counted as study withdrawals because their CPAP usage data were not missing (i.e. CPAP usage of 0 hours/night was objectively evident).
A meta‐analysis examining rates of withdrawals in studies with educational interventions was not performed due to considerable difference in magnitude and direction of effect across the nine studies, preventing meaningful interpretation of study effects (as per section 9.1.4. of the Cochrane Handbook for Systematic Reviews of Intervention) (Table 1).
Low‐certainty evidence (11 studies n = 1702) showed that participants in active supportive interventions were more likely to withdraw from studies when compared to control (OR 1.27, 95% CI 0.97 to 1.66, P = 0.08). This odds ratio translated to an absolute risk increase from 136 to 167 (95% CI: 133 to 208) people per 1000. Evidence in this comparison was downgraded by two levels for imprecision, as OIS criteria were not met, and because the estimate's confidence interval included null (Table 2; Analysis 2.4). Therefore, the overall certainty of the evidence was downgraded by two levels, yielding a 'low' rating.
2.4. Analysis.
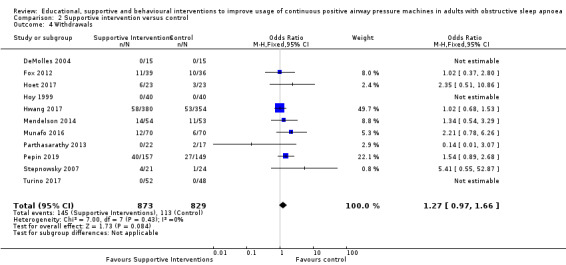
Comparison 2 Supportive intervention versus control, Outcome 4 Withdrawals.
High‐certainty evidence (10 studies, n = 939) showed that participants in active behavioural interventions were less likely to withdraw from studies when compared to control (OR 0.66, 95% CI 0.44 to 0.98, P = 0.04), translating to an absolute risk reduction from 146 to 101 (95% CI: 70 to 143) people per 1000 (Table 3; Analysis 3.4).
3.4. Analysis.
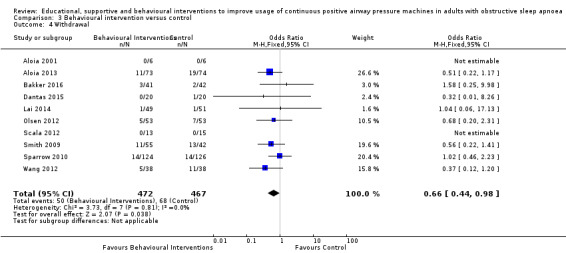
Comparison 3 Behavioural intervention versus control, Outcome 4 Withdrawal.
Very low‐certainty evidence (11 studies, n = 4956) showed that participants in active mixed interventions were less likely to withdraw from studies when compared to control (OR 0.61, 95% CI 0.28 to 1.30, P = 0.20). This translated to an absolute risk reduction from 129 to 83 (95% CI: 40 to 161) people per 1000. Evidence in this comparison was downgraded by three levels due to heterogeneity (I2 = 85%), imprecision (confidence interval includes null and potential for important benefit), and high risk of bias in six studies with a combined analysis weight of 52.0% (Table 4;Analysis 4.4 ).
4.4. Analysis.
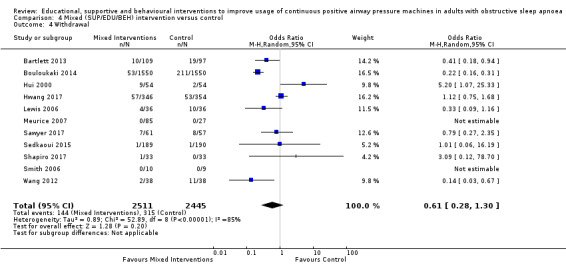
Comparison 4 Mixed (SUP/EDU/BEH) intervention versus control, Outcome 4 Withdrawal.
Daytime sleepiness
Low‐certainty evidence (5 studies, n = 470) demonstrated a non‐significant, decrease in ESS scores (endpoint versus baseline) for participants receiving supportive interventions when compared to control participants (MD ‐0.32, 95% CI ‐1.19 lower to 0.56, P = 0.48). Evidence in this comparison was downgraded by two levels due to high risk of bias associated with non‐masking and subjective outcome measures, in addition to imprecision (OIS criterion not met) (Table 2; Analysis 2.5).
2.5. Analysis.
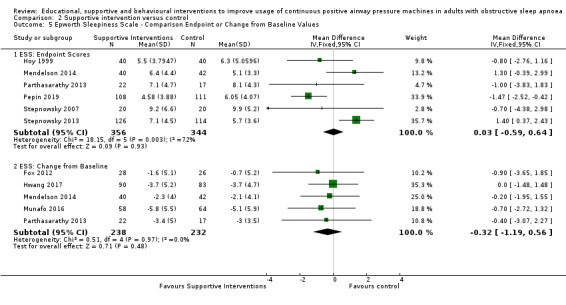
Comparison 2 Supportive intervention versus control, Outcome 5 Epworth Sleepiness Scale ‐ Comparison Endpoint or Change from Baseline Values.
Low‐certainty evidence (5 studies, n = 272) demonstrated a reduction in ESS scores for participants receiving behavioural interventions when compared to control at study endpoints (MD ‐2.42, 95% CI ‐4.27 to ‐0.57, P = 0.01). Evidence in this comparison was downgraded by two levels due to the high risk of bias (associated with unmasked participants and assessors reporting/evaluating subjective outcome measures) and heterogeneity (I2 = 71%) (Table 3; Analysis 3.5).
3.5. Analysis.
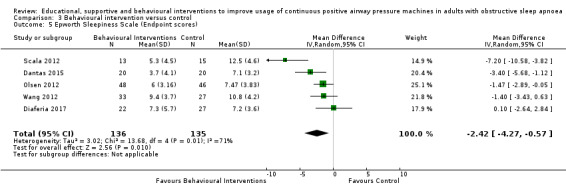
Comparison 3 Behavioural intervention versus control, Outcome 5 Epworth Sleepiness Scale (Endpoint scores).
Meta‐analyses examining ESS scores in studies with educational and mixed interventions were not performed due to considerable difference in magnitude and direction of effect across eligible studies, preventing meaningful interpretation of study effects.
Quality of life
Meta‐analyses examining quality of life scores in studies with educational interventions were not performed as none of the studies included in this comparison used/measured quality of life as a primary or secondary outcome.
Very low‐certainty evidence (3 studies, n = 294) showed that supportive interventions had small positive effect on quality of life scores when measured with the Functional Outcomes of Sleep Questionnaire (FOSQ), FOSQ‐10, Short Form Survey (SF‐36) (change from baseline) (SMD 0.22, 95% CI ‐0.01 to 0.45, P = 0.06). Evidence in this comparison was downgraded by three levels due to high risk of bias associated with subjective outcomes in non‐masked studies, imprecision (OIS criterion not met), as well as due to suspicion of publication bias. (Table 2; Analysis 2.7).
2.7. Analysis.
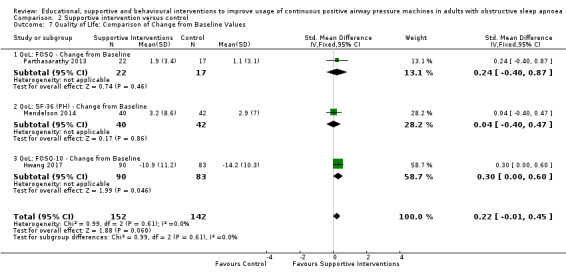
Comparison 2 Supportive intervention versus control, Outcome 7 Quality of LIfe: Comparison of Change from Baseline Values.
Moderate‐certainty evidence (3 studies, n = 228) demonstrated that behavioural interventions had no apparent effect on quality of life scores when measured with the FOSQ, or the physical health portion of the 36‐item Short Form Survey (SF‐36 (PH)) at study endpoints (SMD 0.00, 95% CI ‐0.26 to 0.26, P = 0.98) (Table 3; Analysis 3.7). Evidence in this comparison was downgraded by one level due to high risk of bias associated with non‐masking and subjective outcome measures, yielding a 'moderate' rating.
3.7. Analysis.
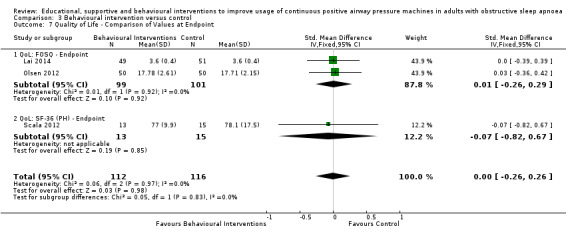
Comparison 3 Behavioural intervention versus control, Outcome 7 Quality of Life ‐ Comparison of Values at Endpoint.
Low‐certainty evidence (2 studies, n = 3012) also showed that mixed interventions had a small and positive effect on quality of life scores when measured with the FOSQ‐10 and SF‐36 (PH) (change from baseline) (SMD 0.45, 95% CI 0.12 to 0.78, P = 0.008). Similarly, when measuring quality of life with the same measures at endpoints only, very low‐quality evidence (4 studies, n = 3191) showed that mixed interventions had a small and positive effect (SMD 0.45, 95% CI 0.06 to 0.83, P = 0.02). Both of these outcomes were downgraded by two levels due to high risk of bias associated with subjective outcome measures in non‐masked studies as well as heterogeneity (I2 = 79%) (Table 4; Analysis 4.5; Analysis 4.6 ).
4.5. Analysis.
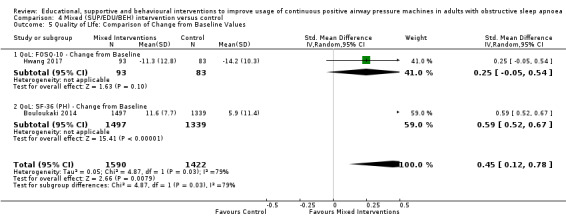
Comparison 4 Mixed (SUP/EDU/BEH) intervention versus control, Outcome 5 Quality of LIfe: Comparison of Change from Baseline Values.
4.6. Analysis.
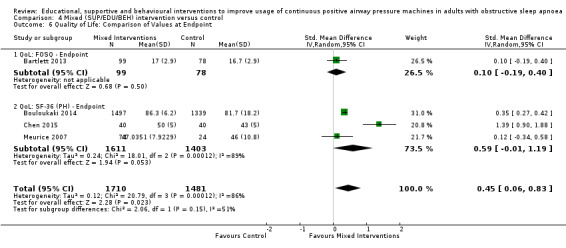
Comparison 4 Mixed (SUP/EDU/BEH) intervention versus control, Outcome 6 Quality of Life: Comparison of Values at Endpoint.
Depressive and anxiety symptom measures
One study (Hoy 1999) showed that a supportive intervention with brief education, two nights of additional titration, and extended home visits decreased anxiety symptom scores when measured with the Hospital Anxiety and Depression Scale (HADS‐A) at six months against control participants (MD ‐1.1, 95% CI ‐2.95 to ‐0.75, P = 0.24). A GRADE assessment was not performed for this outcome as it is a single‐study estimate (Table 2).
Meta‐analyses examining depressive or anxiety symptom scores were not performed for behavioural and educational interventions as none of the studies included in these comparisons reported depression or anxiety as a primary or secondary outcome.
Very low‐certainty evidence (3 studies, n = 333) demonstrated that mixed interventions slightly decreased anxiety scores when compared to control at endpoints when measured with the Depression‐Anxiety Stress Scale (DASS), the Beck Anxiety Inventory (BAI), and the State and Trait Anxiety Inventory (STAI) (SMD =‐0.19, 95% CI ‐0.47 to 0.09, P = 0.18). Evidence in this outcome was downgraded by two levels due to high risk of bias associated with subjective outcome measures in non‐masked studies, and also because a different anxiety symptom scale was used to measure different dimensions of anxiety (e.g. state versus trait). Certainty of evidence was further downgraded by one level due to sub OIS (Table 4; Analysis 4.7). Therefore, the overall certainty of the evidence was downgraded by three levels, yielding a 'very low ' rating.
4.7. Analysis.
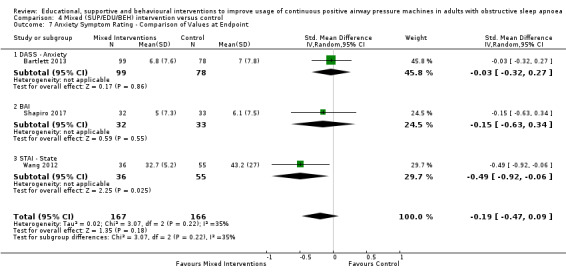
Comparison 4 Mixed (SUP/EDU/BEH) intervention versus control, Outcome 7 Anxiety Symptom Rating ‐ Comparison of Values at Endpoint.
Apnoea hypopnoea index (AHI) on treatment
Two studies reported on AHI (events/hours) following treatment via behavioural interventions (Dantas 2015; Diaferia 2017). Very low‐certainty evidence found a reduction by just under one event per hour when compared to the control group (MD ‐0.95, 95% CI ‐2.25 to 0.34, P = 0.15). Evidence was downgraded by one level due to risk of bias arising from protocol deviation and missing outcome data. Evidence was downgraded by another two levels due to sub optimal information sizes (2 studies, n = 90) and because the estimate's confidence interval included null. No other studies included in this review reported on this outcome (Table 3; Analysis 3.6). Therefore, the overall certainty of the evidence was downgraded by three levels, yielding a 'very low ' rating.
3.6. Analysis.
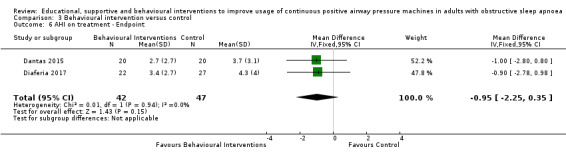
Comparison 3 Behavioural intervention versus control, Outcome 6 AHI on treatment ‐ Endpoint.
Discussion
Summary of main results
This review identified 41 studies assessing behavioural, educational, supportive or mixed strategies for improving continuous positive airway pressure (CPAP) use in 9005 adults with obstructive sleep apnoea (OSA). As a group, behavioural interventions yielded the largest improvements in average nightly CPAP usage when compared to the other intervention classes. Moreover, this class of intervention was the only class to suggest a high degree of certainty and confidence in the estimate, according to our GRADE assessments. Not surprisingly, the mixed interventions were most heterogeneous in design and in effect on CPAP device usage.
Overall completeness and applicability of evidence
Study sites, sample sizes, demographic and clinical characteristics
There was very little variation in participant age, baseline apnoea hypopnoea index (AHI) or baseline Epworth Sleepiness Scale (ESS) across intervention classes (Table 6). The distribution across these demographic and clinical measures likely reflects typical clinical populations.
The behavioural class had a slightly larger proportion of female participants (mean 34.4% female) compared to the other classes (range 24.7% for supportive to 32.4% for mixed). If 0% female assumed for the studies that did not report gender distribution, mean % female ranged from 20.9% (supportive) to 31.3% (behavioural). Importantly, while male gender is an oft‐noted risk factor for OSA and OSA severity, previous authors have suggested that differences in prevalence across genders may be overestimated, and these differences may actually reflect differences in reporting of symptoms across genders and unintentional bias in screening (which is often due to perception of vastly different prevalence rates in itself). Thus, it is difficult to determine an appropriate gender distribution for clinical trials and whether the studies included in this review are reflective of actual clinical populations likely to be targeted by adherence interventions.
It is plausible that interventions directed toward improving CPAP usage are less effective beyond a certain level of pre‐existing compliance. This is supported in part by our planned sensitivity analyses based on control group device usage for some intervention classes. Omitting studies in which average CPAP machine usage was high in control groups (mean ≥ 4 hours/night) had no effect on behavioural educational effect estimates (based on moderate‐ and very low‐quality evidence, respectively), more than doubled the pooled effect estimate for mixed intervention types (very low‐quality evidence), and improved the effect of supportive interventions more modestly, but based on high‐quality evidence. This pattern of findings may suggest that the impact of behavioural and educational interventions may be independent of baseline adherence levels, while mixed and supportive intervention types may be most useful for individuals with low background use. Alternatively, these findings may relate to some other unmeasured difference between studies, study populations or study environments among those with higher versus lower 'background' CPAP usage or may simply be spurious, reflecting the more limited number and lower quality of studies with low use among controls.
See Appendix 1 for further discussion of current evidence pertaining to the impact of CPAP treatment on cardiovascular, cerebrovascular and functional outcomes and the role of CPAP adherence in measuring that impact.
Study duration, intervention duration, contact episodes, contact time
The distribution of intervention duration within each class did not allow subgroup analysis. Therefore, we were not able to establish whether intervention duration is likely to impact CPAP adherence over the short or long term. This unanswered question is relevant to clinical practice, particularly when the cost‐effectiveness of these interventions is considered.
Class‐specific findings
Considerable differences in intervention duration, number of contact episodes, total contact time and study (follow‐up) duration were noted across intervention classes (See Table 7). Supportive and mixed interventions tended to be of longer duration. Number of contact episodes (interquartile range (IQR)) varied from 2 (1 to 5) for educational to 7 (5 to 10) for mixed. Median (IQR) contact time ranged from 45 (12 to 98) for educational to 90 (80 to 240) minutes for behavioural interventions. There was little variability in average study (follow‐up) duration across outcome classes.
There remains a need to assess the impact of intervention on long‐term adherence and outcomes, particularly for patients whose disease is sufficiently severe to warrant intervention but who struggle to persist with CPAP for a number of reasons. More specifically, in addition to assessing the differential impact of interventions based on their class or content, there is a need to better understand which aspects of the intervention structure are most important in assisting patients to initiate CPAP and to become active and engaged partners in their ongoing health care to facilitate long‐term maintenance of treatment. As we discuss in the following sections, it will be important for trialists to: thoughtfully select and report on critical aspects of intervention structure (e.g. duration, number, frequency and duration of clinical contact, timing and sequencing of intervention relative to diagnosis, CPAP titration and CPAP prescription/dispensing). This will improve overall completeness of our corpus of evidence and will permit users of the literature to more adequately assess applicability to their clinical population or setting. It will also enable better cost‐effectiveness estimates.
Qualitative research may assist in identifying common reasons for not persisting with CPAP (e.g. technical problems, insufficient knowledge or understanding of risk and treatment or issues related to motivation, self‐efficacy, or other psychological factors) and quantify their frequency in diverse populations. Such studies will enable better understanding of the mechanisms associated with non‐adherence, elucidate the relationship between initial motivation and ongoing perception of benefit and equip interventional researchers with the means to better determine whether targeting psychological and technical aspects of ongoing CPAP usage modifies long‐term morbidity. The multidimensional nature of CPAP adherence implies that one type of intervention is unlikely to suit all patients, and a personalised approach based on a patient's characteristic and identifiable factors predictive of adherence may be required for some patients. However, such studies may also suggest that a relatively finite set of common factors account for sizeable proportion of variability in adherence. Factors that are both predictive and modifiable represent an appealing target. With this knowledge and with the goal of providing the most cost‐effective treatment, a schedule of adherence interventions may be developed. At one end, low‐intensity, time‐limited, low‐cost and effective interventions may be incorporated into standard management while, at the more intensive end, well‐defined subgroups of non‐adherent patients could be targeted at the outset of CPAP therapy (e.g. after one week of standard care) for more comprehensive or sustained interventions. Between these extremes, more detailed mechanistic information might permit a rational selection of intervention type and duration based on demographic, community or individual clinical factors.
Some of our findings reflect those of previous systematic reviews (Haynes 1996; Haynes 2002a; Haynes 2002b; Haynes 2005; Haynes 2008; Nieuwlaat 2014) of interventions for medication adherence. That is, despite a substantial increase in the number of published studies related to CPAP adherence, the interventions are highly variable and increasingly complex, making it difficult to tease out and evaluate the individual components that may relate most directly to effectiveness. Additionally, the findings of the newer randomised controlled trials (RCTs) only slightly alter the conclusions of the previous version of our review (Wozniak 2014).
Reported endpoints
Interventions also varied substantially in the outcome endpoint measured/reported. Most (29) studies reported CPAP usage outcomes at several endpoints, and only seven studies explicitly identified a primary measurement endpoint. Reported endpoints ranged from one week to two years. A three‐month (or 90‐day) endpoint measurement was available for 20 studies. For the remaining studies, the following endpoints were either the only endpoint reported or the closest to three months: one month (Richards 2007; Shapiro 2017), "1‐2 months" (Chervin 1997), six weeks (Pengo 2018), two months (Dantas 2015; DeMolles 2004; Stepnowsky 2007;), four months (Mendelson 2014; Sedkaoui 2015; Smith 2006; Stepnowsky 2013; Wang 2012), six months (Bakker 2016; Bartlett 2013; Hoy 1999; Lewis 2006; Pepin 2019; Sarac 2017; Scala 2012; Soares‐Pires 2013; Sparrow 2010),and 24 months (Bouloukaki 2014).
Timing of intervention relative to CPAP titration
Not all study authors reported precise timing of CPAP initiation relative to first intervention. Moreover, among those reporting order of CPAP and intervention initiation, many did not specify duration of time between.
Secondary outcomes: health status
Although this review included a number of secondary outcomes related to health status, they were not assessed in the majority of included studies. The most commonly‐measured secondary outcome across intervention classes was daytime sleepiness using ESS. Due to heterogeneity in direction of effect for some classes (educational and mixed), meta‐analyses could not be performed. Among classes for which analysis was appropriate, meta‐analysis included: five behavioural interventions reporting baseline/endpoint scores (Dantas 2015; Diaferia 2017; Olsen 2012; Scala 2012; Wang 2012), and five supportive interventions reporting change from baseline scores (Fox 2012; Hwang 2017; Mendelson 2014; Munafo 2016; Parthasarathy 2013).
Quality of the evidence
Several issues affect the reliability of our findings and their applicability to the general OSA population. Across classes, we downgraded the evidence primarily for risk of bias (behavioural, educational, supportive, mixed) and inconsistency (educational, mixed) ('Summary of findings' tables 1 to 4). Performance bias due to lack of blinding is likely for subjective‐ and observer‐rated outcomes and is likely to affect all the studies in this review. Across all four classes, statistical variation between studies may be attributable to one or more plausible causes, including different populations recruited, variation in the modalities of interventions provided or differences in the timing or intensity of interventions.
Educational interventions were downgraded for both risk of bias and inconsistency across all outcomes. Among supportive interventions, most outcomes were downgraded for risk of bias and several (N deemed adherent, withdrawals, ESS, quality of life (QoL)) for imprecision. Among behavioural interventions, we downgraded the evidence primarily for high risk of bias. ESS was additionally downgraded for inconsistency and AHI for imprecision. Among mixed interventions, nearly all outcomes were downgraded for risk of bias and consistency and some for imprecision.
With the exception of behavioural interventions (where I2 = 0%), there was substantial statistical heterogeneity in CPAP usage effect estimates across studies within each class: I2 = 66% for educational, I2 = 42% for supportive and I2 = 92% among mixed interventions. In both supportive and educational interventions, heterogeneity was attributable to effects of one or two studies. In the supportive intervention class, heterogeneity derived from the single study with results favouring the control arm (Mendelson 2014). Among educational interventions, heterogeneity may be largely attributable to differences in magnitude of effect. Among mixed interventions, heterogeneity could not be easily accounted for, which is not surprising given the heterogeneous nature of the interventions within the 'mixed' category.
Post‐hoc subgroup analyses
Given the extensive variability observed in intervention duration, number of intervention episodes, and intervention contact time across studies, we conducted exploratory post hoc subgroup analyses comparing subgroups of studies based on these dimensions, which may collectively relate to intervention intensity. In post hoc exploration of results, we hypothesised that intervention intensity may help to predict effectiveness. Specifically, for the behavioural, educational, and mixed classes, we examined each of the following subgroups: intervention duration > 4 weeks, intervention episodes > 1 and intervention contact time > 60 minutes. For each, we observed any change in effect estimate and, where relevant, heterogeneity (I2). For supportive interventions, only intervention duration was consistently reported, but we also elected to examine another putative aspect of intensity for that class of intervention: the extent to which the supportive contacts were administered by computer/automated messaging (versus by human contact) and whether the contact frequency/interval was pre‐determined. As such, for the supportive intervention class, we examined a subgroup comprising interventions in which the intervention involved human (rather than purely automated) support and a subgroup comprising interventions that involved scheduled (as opposed to ad hoc) human support. Below, we report the effect estimates (95% CIs), heterogeneity (I2) values for each post hoc subgroup analysis, and tests for subgroup differences.
In summary, we did not find evidence of substantial and consistent effects of any proposed dimension of intervention intensity on CPAP usage across intervention classes in post hoc exploratory subgroup analyses. For behavioural interventions (Analysis 6.8) and educational interventions (Analysis 6.2), the difference between a single contact episode and more than one contact episode (irrespective of duration) was not substantial, while mixed interventions that included greater than one contact episode showed a larger estimated effect on CPAP usage (Analysis 6.11). Importantly though, only one mixed intervention study (Bartlett 2013) had a single contact episode (Hwang 2017 number of contact episodes unknown; Lewis 2006 did not report SDs), so this must be interpreted conservatively. The respective treatment effects were (MD 0.98, 95% CI 0.32 to 1.63; participants = 4036; studies = 9; I2 = 91%) for those with > 1 episode (Bouloukaki 2014; Chen 2015; Hui 2000; Lewis 2006; Meurice 2007; Sawyer 2017; Sedkaoui 2015; Shapiro 2017; Wang 2012) versus (MD ‐0.60, 95% CI ‐1.33 to 0.13; participants = 206; studies = 1; I2 = 0%) for the single study with only a single contact episode (Bartlett 2013), with a demonstrated subgroup difference (Chi² = 9.94, df = 1 (P = 0.002), I² = 89.9%).
6.8. Analysis.
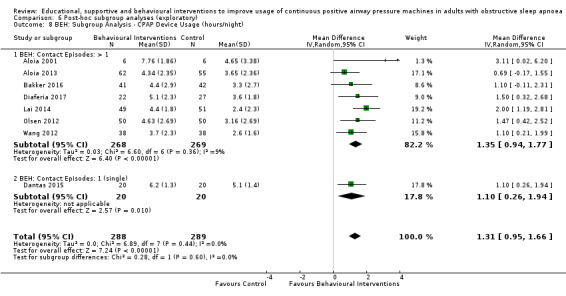
Comparison 6 Post‐hoc subgroup analyses (exploratory), Outcome 8 BEH: Subgroup Analysis ‐ CPAP Device Usage (hours/night).
6.2. Analysis.
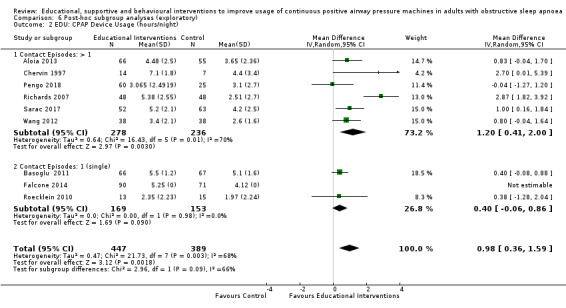
Comparison 6 Post‐hoc subgroup analyses (exploratory), Outcome 2 EDU: CPAP Device Usage (hours/night).
6.11. Analysis.
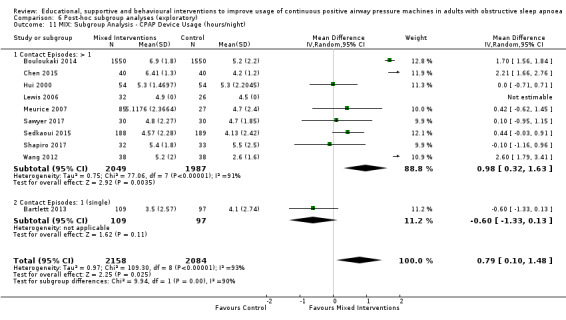
Comparison 6 Post‐hoc subgroup analyses (exploratory), Outcome 11 MIX: Subgroup Analysis ‐ CPAP Device Usage (hours/night).
Intervention duration > 4 weeks appeared to have similar effect to shorter (≤ 4 week) duration among behavioural interventions (Analysis 6.7). Educational interventions > 4 weeks (Hwang 2017; Pengo 2018; Wang 2012, mean 10 weeks) and supportive interventions > 12 weeks (Hoy 1999; Mendelson 2014; Parthasarathy 2013; Pepin 2019, mean 17.8 weeks) showed no certain improvements in adherence. Shorter duration subgroups in these classes appeared to have a larger effect estimates, however testing for subgroup differences in both intervention categories showed no certain difference; educational (Aloia 2013; Basoglu 2011; Chervin 1997; Falcone 2014; Richards 2007; Roecklein 2010; Sarac 2017, mean 1 week): (MD 1.20, 95% CI 0.39 to 2.01; participants = 675; studies = 7; I2 = 75%), (Chi² = 3.36, df = 1 (P = 0.07), I² = 70.2%) (Analysis 6.1), supportive (Chervin 1997; DeMolles 2004; Fox 2012; Hoet 2017; Hwang 2017; Munafo 2016; Stepnowsky 2007; Stepnowsky 2013; Turino 2017, mean 9.9 weeks): (MD 0.72, 95% CI 0.43 to 1.01; participants = 896; studies = 9; I2 = 0%), Chi² = 0.17, df = 1 (P = 0.68), I² = 0%) (Analysis 6.4). For mixed interventions, longer duration (Bouloukaki 2014; Chen 2015; Hui 2000; Hwang 2017; Lewis 2006 (not included in estimate); Meurice 2007; Sedkaoui 2015; Wang 2012) may have illicited a larger effect estimate (MD 1.22 (0.60‐1.83)), compared to shorter duration (Bartlett 2013; Sawyer 2017; Shapiro 2017) (MD ‐0.31, 95%CI ‐0.83 to 0.21; participants = 331), (Chi² = 13.79, df = 1 (P = 0.0002), I² = 92.7%) (Analysis 6.10).
6.7. Analysis.
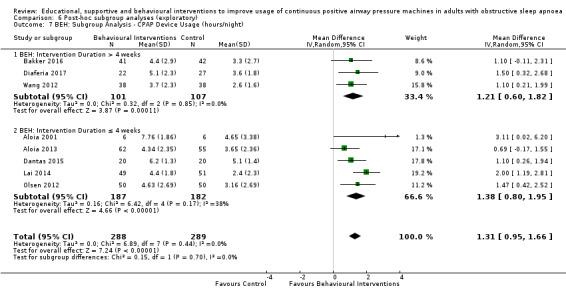
Comparison 6 Post‐hoc subgroup analyses (exploratory), Outcome 7 BEH: Subgroup Analysis ‐ CPAP Device Usage (hours/night).
6.1. Analysis.
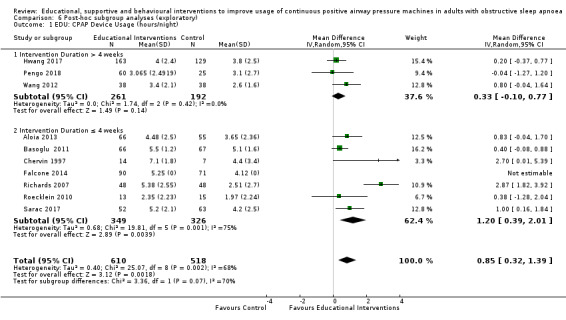
Comparison 6 Post‐hoc subgroup analyses (exploratory), Outcome 1 EDU: CPAP Device Usage (hours/night).
6.4. Analysis.
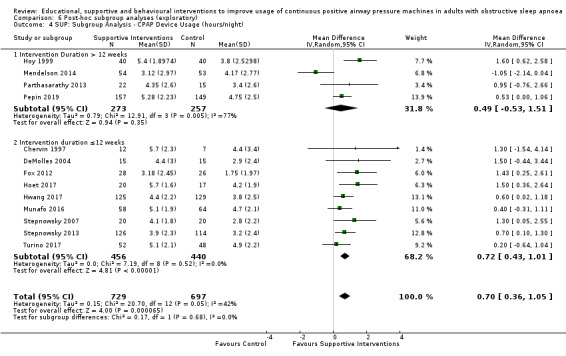
Comparison 6 Post‐hoc subgroup analyses (exploratory), Outcome 4 SUP: Subgroup Analysis ‐ CPAP Device Usage (hours/night).
While behavioural interventions with contact time > 60 minutes had lower effect estimates than those with shorter contact times, only two small studies were in the former subgroup and confidence intervals overlapped substantially (Analysis 6.9) . For educational interventions, three studies had > 60 minutes of contact time and the estimated effect for this subgroup appeared to be larger than interventions with ≤ 60 minutes of contact, however a subgroup difference was not shown (Analysis 6.3). For mixed interventions, there may be a difference between subgroups (Hwang 2017 excluded due to unknown contact time) (MD 1.45, 95% CI 0.73 to 2.16; participants = 3751; studies = 6; I2 = 91%) for those with > 60 minutes contact time (Bouloukaki 2014; Chen 2015; Sawyer 2017; Sedkaoui 2015; Wang 2012) versus (MD ‐0.15, 95% CI ‐0.56 to 0.27; participants = 491; studies = 4; I2 = 0%) for those with ≤ 60 minutes (Bartlett 2013; Hui 2000; Meurice 2007; Shapiro 2017), (Chi² = 14.14, df = 1 (P = 0.0002), I² = 92.9%) (Analysis 6.12).
6.9. Analysis.
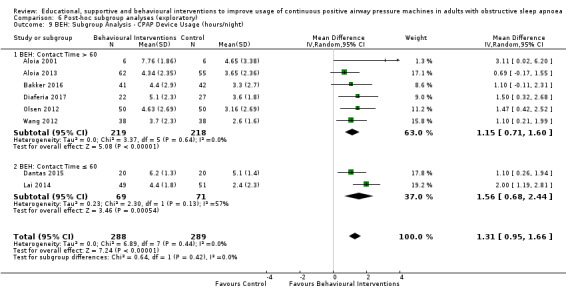
Comparison 6 Post‐hoc subgroup analyses (exploratory), Outcome 9 BEH: Subgroup Analysis ‐ CPAP Device Usage (hours/night).
6.3. Analysis.
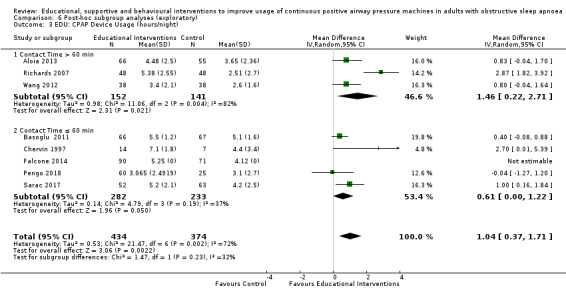
Comparison 6 Post‐hoc subgroup analyses (exploratory), Outcome 3 EDU: CPAP Device Usage (hours/night).
6.12. Analysis.
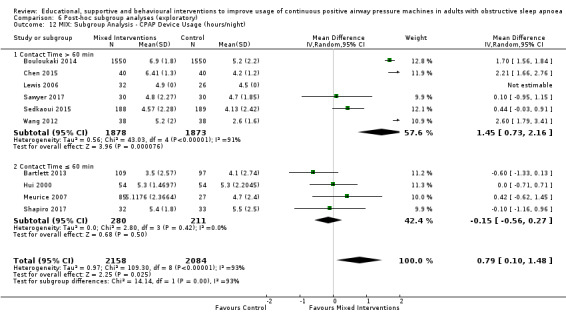
Comparison 6 Post‐hoc subgroup analyses (exploratory), Outcome 12 MIX: Subgroup Analysis ‐ CPAP Device Usage (hours/night).
Finally, our post hoc analysis examining differences in delivering supportive interventions through human interaction versus automated intervention demonstrated uncertain results (Analysis 6.5). It appeared however,supportive interventions prescheduled human support appeared to have a greater effect estimate (MD 1.43 (0.61 to 2.24) I2 = 0%) compared to interventions with no scheduled human support ( MD 0.58 (0.33 to 0.83) I2 = 45%), ( Chi² = 3.82, df = 1 (P = 0.05), I² = 73.8%) (Analysis 6.6.) Moreover, limiting to interventions involving human support substantially reduced the observed heterogeneity of effects among supportive interventions.
6.5. Analysis.
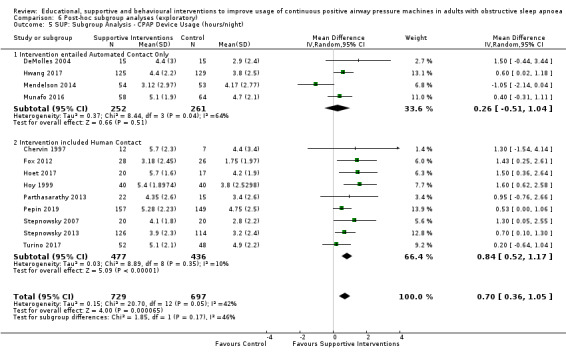
Comparison 6 Post‐hoc subgroup analyses (exploratory), Outcome 5 SUP: Subgroup Analysis ‐ CPAP Device Usage (hours/night).
6.6. Analysis.
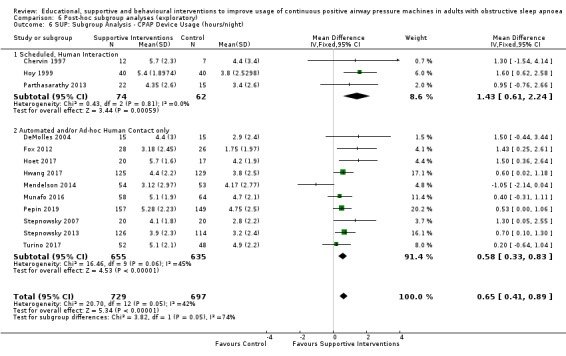
Comparison 6 Post‐hoc subgroup analyses (exploratory), Outcome 6 SUP: Subgroup Analysis ‐ CPAP Device Usage (hours/night).
Post‐hoc sensitivity analyses
To assess effect of excluding studies with high risk of bias
We performed sensitivity analyses (Table 12), excluding studies with high risk of bias from each class to determine the extent to which our effect estimates may have been influenced by lower quality studies. Omission of studies with high risk of bias resulted in a reduction of the estimated improvement in CPAP usage for behavioural interventions, but statistical significance was retained (MD 1.05, 95% CI 0.57 to 1.53; participants = 340; studies = 4; I2 = 0%). Interestingly, for educational (MD 0.98, 95% CI 0.07 to 1.89; participants = 642; studies = 4; I2 = 86%) and supportive (MD 0.75, 95% CI 0.42 to 1.09; participants = 728; studies = 5; I2 = 34%) interventions, omission of high risk of bias improved effect estimates slightly while retaining statistical and clinical significance. Due to high heterogeneity and more pronounced differences in direction (two of five studies with mean differences favouring controls, I2 = 92%) and magnitude of effect, this sensitivity analysis was not performed for mixed intervention class.
To assess effect of differences in updated review intervention classification
We also performed sensitivity analyses (Table 15) to determine if the approach employed for classifying interventions in the current review update, relative to the original classification decisions (Wozniak 2014), substantially affected the results obtained for the original three intervention classes (i.e. behavioural, educational and supportive). To perform these sensitivity analyses, any study that was included in the original review was assigned to the class assigned in the original review. Any study that was new to the updated review retained the class assigned in the update. For behavioural interventions, three studies were differentially classified (Wang 2012 classified as behavioural in our update but as educational in original review; Richards 2007 and Roecklein 2010 classified as educational in our update but as behavioural in the original review). Results showed (MD 1.47, 95% CI 1.12 to 1.83; participants = 625; studies = 9; I2 = 48%) that the original classification schema would have improved the estimated effect relative to our reported results.
Sensitivity analysis of educational interventions according to the original class allocation (MD 0.48, 95% CI 0.21 to 0.76; participants = 1095; studies = 8; I2 = 0%) resulted in a reduction of the effect estimate, but retained significance. Relative to our classification, the original classification reduced the number of studies in the educational class from 10 to 8, entailed differential allocation of three studies and reduced the heterogeneity to 0%.
Classification of supportive interventions according to the original review (MD 0.58, 95% CI 0.36 to 0.81; participants = 1534; studies = 14; I2 = 45%) resulted in a reduction in the estimated effect and also retained significance, favouring the intervention. Heterogeneity was essentially unchanged.
To assess effect of endpoint selection
Sensitivity analyses to examine whether the endpoint measured/reported by study authors (closest endpoint to three months post‐intervention, the modal endpoint) affected the effect estimates. A summary of results is provided (Table 16).
Treatment fidelity
Treatment fidelity, which can be defined as strategies that monitor and enhance the accuracy and consistency of an intervention provided, is of particular importance in behavioural studies. Assessment of treatment fidelity is required to ensure validity of study outcomes. In the current review,six6 studies (Aloia 2013; Bakker 2016; Lai 2014; Sawyer 2017; Shapiro 2017; Olsen 2012) implemented treatment fidelity checks, and the lack of checks in other studies is a potential source of inconsistency between studies.
Potential biases in the review process
Two potential sources of bias have been identified in our review process. First, the categorisation of studies in this review is based on our assessment of the core attributes of the intervention, based on the authors' descriptions within Methods sections of the published reports, and how it differed from the control group (i.e. the 'net' intervention). It is possible that our classification of studies by intervention type is itself a crude mean of differentiating between the interventions or other differentiating features (e.g. intervention intensity) may ultimately prove more important in defining and comparing classes of interventions. Furthermore, the addition of a 'mixed' intervention type to this updated review reduced the imprecision of assigning interventions with mixed components to one class arbitrarily, but also introduced a new highly‐heterogeneous category. We found no evidence to suggest that our classification procedures biased our most important results in favour of the intervention. Sensitivity analysis examining results for behavioural interventions using the original versus our updated classification scheme showed that, if anything, our classification approach was more conservative for this outcome. Differences in classification for educational and supportive interventions did produce nominally more favourable results in the current review and does suggest that classification decisions can impact results. Overall, our classification procedures did not appear to impact heterogeneity. Relative to the allocation implied under original classification procedures, our behavioural class was less heterogeneous (0% versus 48%), our educational class was more heterogeneous (66% versus 0%) and our supportive class heterogeneity was essentially equivalent (42% versus 45%).
Second, we did not assess how 'active' components of control interventions may have confounded the results of some of the studies. Many of the control group interventions in the included studies attempted to inform participants about OSA and the importance of treatment through written materials, videos or sessions with specialist staff. However, what constitutes usual care varied between treatment centres. For example, the control groups of Hoy 1999 and Hui 2000 received education and support at least equivalent to that received by the intervention group in Chervin 1997. Some studies attempted to balance contact with participants between intervention and control groups or to provide 'placebo' in the control arms. In other studies, given the nature of the interventions, this was not practical. We did attempt to mitigate the effect of active controls by including only studies in which the intervention arm(s) received the same 'background' level of intervention as the control. However, the varied intensity of the background or control intervention, in addition to the 'net intervention' within the studies, could have influenced effect sizes in our analyses.
Agreements and disagreements with other studies or reviews
To our knowledge, other than the previous version of this review (Wozniak 2014), no other published reviews that meet the standard criteria of a systematic review have investigated the role of educational, supportive or behavioural interventions in improving adherence to CPAP.
Authors' conclusions
Implications for practice.
Any type of educational, supportive or behavioural intervention (beyond usual care) is likely to improve continuous positive airway pressure (CPAP) usage by approximately one hour. In CPAP‐naive people with severe sleep obstructive sleep apnoea (OSA), very low‐quality evidence indicates that supportive and mixed‐type interventions increase usage compared with usual care. Moderate‐quality evidence shows that a short‐term educational intervention results in a modest increase in CPAP usage. High‐quality evidence suggests that interventions employing active, motivational, cognitive and behavioural strategies, requiring interactive engagement by participants (i.e. those interventions broadly categorised as 'behavioural' in the current review) leads to a relatively large and clinically‐significant increase in CPAP machine usage (hours per night).
For all but behavioural interventions, risk of bias and inconsistency in size or direction of effect across studies introduced moderate (supportive) to substantial (educational, mixed) uncertainty in the size of the difference that might be anticipated in practice for CPAP usage outcomes. We acknowledge that our assessment of risk of bias was based primarily on published reports and, only infrequently, on additional information obtained from study authors. Thus, it is possible that some judgements were based on incomplete information. However, we believe it is most prudent to rely on the published record of study procedures rather than on recollections of authors, often many years after study completion, particularly as such information was not subject to peer review.
Implications for research.
The evidence assembled in this review provides a useful framework for additional research. Investigators should bear in mind the following considerations in developing further studies of CPAP adherence interventions to address uncertainties.
Results of our post hoc analysis indicate that further research to determine who would benefit most from these interventions is warranted. Recruitment of patients with previous OSA diagnosis, especially those who have not successfully persisted with treatment, is important.
Patients with milder OSA should be recruited to future trials because they may be less likely to persist with treatment if they do not perceive symptomatic benefit and, without treatment, they may ultimately progress to more severe OSA.
Reasons why participants leave studies should be documented to obtain information on whether and how different types of interventions modify perception of benefit or the balance of benefit and side effects.
How missing values are handled or incorporated into statistical analyses should be explicitly described to enable testing of the sensitivity of effect estimates through different approaches to adjust for the missing data. Many authors report that analyses were by, for example, intent‐to‐treat (ITT) analysis, but did not specify whether missing data values were excluded or imputed.
Validated instruments have been developed for assessing quality of life and symptoms in people with OSA. Trialists should consider using these measurements to explore whether improved adherence affects these outcomes within the time frame of the intervention. Depending upon duration of the study or follow‐up period, consideration should be given to incorporating measures of potentially related health outcomes (e.g. cardiovascular, cerebrovascular, cognitive functional measures, motor vehicle incidents).
Long‐term assessment should examine the duration and durability of improvements in CPAP adherence, as well as cost‐effectiveness.
Few studies measured quality of life, depressive or anxiety symptoms. There is growing evidence from cohort studies (Chirinos 2017; Ohayon 2003; Saunamaki 2007) as well as randomised controlled trials (RCTs) and RCT meta‐analyses (McEvoy 2016; Zheng 2019) demonstrating that CPAP use is associated with improvements in quality of life, depressive and anxiety symptoms.
Involvement of bed partners would help us to understand what role they may play in improving the use and long‐term uptake of CPAP.
It remains uncertain what intensity of intervention is required to effect behavioural change and this should be a focused area of study. A common metric for intervention intensity should be derived in which various parameters of intensity (e.g. duration of intervention, number and frequency of contact episodes, total intervention contact time, contact time per contact episode) are defined, and used to develop, characterise and report on interventions. Studies with educational and behavioural interventions with variation across such well‐defined parameters would be helpful in elaborating this area further.
More head‐to‐head comparisons of different approaches are needed.
Cost‐effectiveness research would help to establish how resources can best be allocated in implementing these interventions. Ideally, studies with multiple intervention arms that vary in level of intensity across well‐defined parameters (and that incorporate baseline measures of participant OSA/CPAP knowledge, motivation, self‐efficacy and outcome expectations) would address several important remaining questions:
whether there is a minimal baseline intensity of intervention that the majority of patients are likely to require, regardless of individual knowledge, motivation or self‐efficacy,
which parameters of intervention intensity are most relevant to improving CPAP adherence, and
whether baseline knowledge, motivation and self‐efficacy is related to the intensity of intervention required for improvements in CPAP adherence.
Personalised interventions may be appropriate for some individuals but may not be required for all new CPAP users. Given the effectiveness of behavioural interventions, it may be most cost‐effective to consider personalised interventions only for those patients who are treatment‐resistant after behavioural interventions fail to optimise adherence.
Treatment fidelity should be measured in studies incorporating behavioural interventions to ensure the validity of treatment outcomes.
Future systematic reviews could usefully consider the validity of intervention classes along the lines identified in this review.
It is not clear whether RCTs of CPAP adherence interventions will be the best study design for assessing the impact of improved CPAP adherence on downstream health outcomes. However, high‐quality, long‐term studies are needed to rigorously assess the impact of nightly CPAP dosage and treatment duration on the nature and time course of improvements across many symptom domains (sleepiness, psychological symptoms, quality of life, and social and occupational functioning), and other clinically‐important health outcomes (cardiovascular markers, cognitive/neurological impairment and functioning). Very importantly, particularly for studies attempting to measure the impact of CPAP usage on reversing chronic disease risk and sequelae: such studies will not only need to be of sufficient length such that reversal of disease/functional markers would be rationally expected, but these studies need to ensure that participants in the intervention arms achieve sufficient nightly CPAP usage to warrant robust conclusions. By way of analogy, if, in the majority of pharmaceutical trials, participants in the intervention arms received, on average, only half of the therapeutic dose of medication, we would be reluctant to draw conclusions as to the effectiveness of the medication. (See section below for further discussion). Future RCTs seeking to evaluate the impact of CPAP on health outcomes should consider including interventions to optimise CPAP adherence (i.e. to achieve nightly use of six to eight hours/night) as an integral part of their protocols so as not to waste valuable resources to assess the impact of sub optimal dosing.
See Appendix 1 for further discussion of current evidence pertaining to the impact of CPAP treatment on cardiovascular, cerebrovascular and functional outcomes and the role of CPAP adherence in measuring that impact.
Final considerations: other potential confounding factors and conclusions derived from RCTs
Though the advent of technology to directly measure hours of adherence has been invaluable to the field, any study seeking to evaluate the short‐ and long‐term health impact of CPAP will need to simultaneously measure total sleep duration in order to draw accurate conclusions from the results. For the same reason that early studies relying on subjective CPAP usage reports are known to be biased, reliance on CPAP device recordings also has an implicit bias: the duration of sleep without CPAP is generally unknown. It may be reasonably safe to assume that the closer the CPAP device‐recorded sleep time is to eight hours, the lower the likely duration of unrecorded sleep time. That said, confirmation would require direct observation or other means of sleep recording. For recorded sleep durations less than eight hours, there are several additional factors that may confound the interpretation of results.
First, the fewer hours recorded, the greater the likelihood and duration of unrecorded sleep time. As such, the mean CPAP usage (e.g. mean 3.67 across published RCTs in a recent review (Yu 2017)) suggests that a substantial proportion, if not the majority, of sleep time across studies was unrecorded (and therefore, untreated). Under these conditions, the sleep during which OSA remains untreated is, on average, exerting a greater influence on the measured health outcomes than the smaller proportion of the night in which the OSA is treated. For example, a patient diagnosed with 'mild' OSA based on an average apnoea hypopnoea index (AHI0 = 10 who uses CPAP at optimal pressure for six of eight hours of sleep (achieving an AHI = 0 for 6 of 8 hours), will still have an AHI=10 during the remaining two hours. Assuming apnoeic/hypopnoeic events are evenly‐distributed throughout all sleep stages and positions, this would reduce overall AHI to 2.5, but will not change the AHI during any period of non‐use. A patient with more severe OSA (e.g. AHI = 35) would need to use CPAP at effective pressure for seven of eight hours per night in order to reduce overall AHI below diagnostic threshold and would need to adhere for > 4.5 hours/night to result in an overall (i.e. full night) AHI within the 'mild' range of severity (AHI < 15).
Second, the device records time that the mask is on and does not distinguish between wake, sleep or specific sleep stages. This has several potential implications for interpreting results of CPAP‐effectiveness studies. To the extent that a patient's OSA is sleep stage‐related or positional, the actual AHI during the latter hours of sleep may be markedly worse than earlier hours. And, because patients are more likely to comply during earlier as opposed to later segments of sleep, unrecorded sleep time may comprise a disproportionate amount of REM (rapid eye movement sleep) and, depending on the total duration of sleep and sleep onset latency, a substantial proportion of deep (i.e. slow wave) sleep. Therefore, to the extent that unrecorded sleep time comprises REM, deep sleep or supine position, CPAP usage time may be poorly associated with a variety of important health outcomes in studies wherein most participants have low CPAP adherence.
Third, without knowing the duration of unrecorded/untreated sleep, the total sleep duration among participants is unknown and unaccounted for in most studies. Even apart from the deleterious health consequences of untreated OSA, the health effects of chronic sleep deficiency or insufficiency are well‐established. Thus, failure to achieve recommended nightly sleep duration among some (perhaps many) study participants within these RCTs could contribute to the absence of apparent benefits of CPAP on cardiovascular (CV) outcomes.
Finally, accumulated RCT evidence addressing the role of CPAP on CV risk derives from studies of short and intermediate duration (i.e. majority of studies are < 1 year follow‐up duration). This may be an insufficient time frame, particularly at sub therapeutic treatment dosages, to draw meaningful conclusions about the impact of CPAP treatment on CV risk.
In conclusion, while current evidence strongly suggests that there are limited (if any) cardiovascular benefits of CPAP when used at or below commonly‐employed research thresholds of adherence, extant evidence does not address the potential for improvement in CV risk at CPAP usage thresholds beyond four hours/night or beyond one year of CPAP treatment. Moreover, these studies do not control for the confounding effects of total sleep duration or duration of untreated sleep. Each of these factors has the potential to substantially influence conclusions about the health benefits of CPAP use and, therefore, about the importance of optimising CPAP adherence. As such, it is difficult to determine how best to allocate resources for expanding or improving CPAP adherence interventions at this time. At a minimum, such decisions will require that studies be undertaken to establish the health benefits of CPAP, when used at optimal therapeutic dosage (i.e. throughout sleep). Without this information, we run the risk of erroneous conclusions regarding the reversibility of OSA sequelae or of the effectiveness of our current gold‐standard OSA treatment. Should such studies demonstrate substantial cardiovascular, cerebrovascular or other health benefits, this would suggest that rather considerable resources should be devoted to developing interventions to optimise CPAP adherence. On the other hand, should such studies show that even optimal CPAP usage is insufficient to reverse these important OSA‐linked health risks and outcomes, resource expenditures should be differentially allocated. To optimise CPAP use for improvement of OSA symptoms, depressive symptoms and quality of life (QoL), a small number of additional studies may be warranted to determine how different CPAP adherence intervention classes may be better targeted or personalised for different patient subgroups. On the other hand, to address OSA‐related cardio/cerebrovascular health outcomes, such findings would suggest that resources would be more optimally targeted at chronic disease prevention.
What's new
| Date | Event | Description |
|---|---|---|
| 29 April 2019 | New citation required and conclusions have changed | Review updated with 11 new studies. The background was redrafted and updated. 'Risk of bias 2' assessment of the primary outcome for all studies. Outcomes and subgroup analyses updated. |
| 29 April 2019 | New search has been performed | New literature search run |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 17 January 2013 | New citation required and conclusions have changed | 13 new studies added. Changes made in review conclusions in relation to short course interventions. Summary of findings table added |
| 17 January 2013 | New search has been performed | Literature searches rerun |
| 10 March 2009 | Amended | Spelling correction |
| 5 September 2008 | Amended | Converted to new review format. |
| 12 October 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The Background and Methods section of this review are based on a standard template used by Cochrane Airways.
We are very grateful to the Cochrane Airways Group, Elizabeth Stovold, Christopher J. Cates, and Toby J. Lasserson for providing ongoing support with searching for and retrieving studies for inclusion in the review, statistical analysis, and 'Risk of bias' assessment processes. We are also grateful for to the study authors who responded to our requests for additional data and information for this version of the review: Jessie Bakker (Bakker 2016), Jean Louis Pépin (Mendelson 2014), Daniela Scala (Scala 2012), Vito Falcone (Falcone 2014), Amy Sawyer (Sawyer 2017), Delwyn Bartlett (Bartlett 2013), Ronald Chervin (Chervin 1997), and Carol Smith (Smith 2006). We additionally acknowledge Massimo Attanasio and Adolfo Tambella for their translation of non‐English study articles (Scala 2012). Lastly, we acknowledge the contribution of Vidya Nadig to a previous version of this review (Smith 2009b).
The authors and Cochrane Airways are grateful to the following peer and consumer reviewers for their time and comments:
Martino F. Pengo, IRCCS Istituto Auxologico Italiano, Italy;
John Fleetham, The University of British Columbia, Canada;
José‐Ramón Rueda, University of the Basque Country, Spain;
Euphrasia Ebai‐Atuh Ndi (consumer), Cameroon;
Ozen K Basoglu, Ege University Faculty of Medicine, Turkey; and
Keith Wong, The University of Sydney, Australia
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Impact of CPAP adherence on assessment of health outcomes
Despite persuasive evidence for several health benefits of CPAP treatment from longitudinal studies, the largest RCT of CPAP and secondary prevention was negative with no apparent benefit of CPAP therapy in reducing the incidence of cardiovascular events (McEvoy 2016 ). While the authors argued that low adherence was insufficient to explain the lack of benefit, we would suggest that further consideration may be warranted given the many downstream implications of these conclusions. Specifically, if there is widespread agreement in the field that there are limited cardiovascular, cerebrovascular and mortality benefits to using CPAP, then this has implications for future expenditure, not only on RCTs targeting these outcomes with CPAP, but also on development and assessment of CPAP adherence interventions, the topic addressed in the present review. We believe that the data do not sufficiently support the negative conclusions in recent reports; rather, we would suggest that further study is definitely warranted to achieve the robust results required for directing future research and clinical initiatives in this field.
In McEvoy 2016 RCT, the average nightly CPAP use was 3.3 hours. Interestingly, while 58% of participants used CPAP for < 4 hours/night, the authors' propensity‐matched analyses comparing 'good' (> 4 hours/night) to 'bad' (< 4 hours/night) users, showed marginal benefit of CPAP usage for stroke risk and significant benefit for risk of composite cerebrovascular events. We would suggest that this is somewhat remarkable given the rather low 'adherence' definition employed. That is, considering that a cut‐off of 4 hours/night has no sound evidentiary basis and would not result in subclinical OSA with treatment (i.e. would not reduce the average participant's AHI to less than 15 events/hour, below 'moderate OSA' diagnostic threshold), finding any effect is noteworthy. Additionally, despite low overall adherence among participants, the study demonstrated significant differences in many secondary measures (sleepiness, depression, QoL measures, work absenteeism) which supports previous evidence that different benefits may accrue at different adherence levels (Campos‐Rodriguez 2005; Sawyer 2011; Stradling 2000; Wang 2017; Weaver 2007; Zimmerman 2006).
Interestingly, in their sample size calculations, the SAVE trial authors found that CV risk increased by 25% to 32% for every increase of 10 events/hour (AHI). Given that CPAP corrects AHI (in the majority of users) during use (but not during non‐use), we can calculate the expected improvement in AHI for the participants in the study. That is, 3.3 hours/night average use would reduce AHI among participants in the CPAP arm from the baseline average AHI of 29 events/hour, to a weighted mean endpoint AHI of 15.6 events/hour (assuming 8 total hours sleep, AHI = 0 for 3.3 hours sleep with CPAP use and AHI = 29 during remaining 4.7 hours without CPAP use). By investigators CV risk calculations, this would correspond to the average 'treated' CPAP arm participant having a roughly 37% to 47% elevated CV risk due to 'residual' OSA (i.e. risk attributable to proportion of sleep without CPAP), relative to OSA‐free population. This suggests that the apparent lack of effect of CPAP use on CV risk may be due to insufficient reduction of overall AHI (i.e. inadequate OSA treatment) among participants in the 'treatment' arm due to sub therapeutic CPAP adherence. In other words, rather than providing evidence for a lack of benefit of CPAP on CV outcomes, the findings may be interpreted as follows:
a) adherence of 3.3 hours/night is insufficient to produce meaningful benefit in CV risk or MVA risk;
b) modest (3.3 hours/night) adherence yields statistically‐significant benefits for other outcomes including daytime sleepiness, depression/anxiety, QoL measures, and work absenteeism;
c) the widespread and arbitrary threshold of ≥4 hours/night warrants closer scrutiny and may be contributing to underestimation of the impact of CPAP on CV risk and
d) as with any pharmacologic treatment, studies should report CPAP dosage and present aggregate findings based on different dosage thresholds to facilitate appropriate interpretation.
Additionally, in the largest and most recent systematic review and meta‐analysis of the effect of CPAP on cardiovascular outcomes (Yu 2017), authors included data from 10 trials (9 CPAP; 1 ASV) of 7266 patients with sleep apnoea and found no significant association with major CV events, CV death or all‐cause death. Meta‐regressions identified no associations of PAP with outcomes for different levels of OSA severity, follow‐up duration, or adherence to PAP (all P > .13). Failure to observe an impact on CV outcomes in these RCTs may again relate to poor PAP adherence observed across all included RCTs, rates likely consistent with clinical populations more generally. The vast majority of (and all large) RCTs analysed in the review have mean/median CPAP usage < 4 hours/night. A single medium‐sized study (Barbé 2012) and three small studies had mean nightly use > 4 hours/night. By our calculations, the weighted average nightly CPAP usage across all studies was 3.67 hours/night and 83.6% of studies had mean/median < 4 hours/night. We would recommend caution when interpreting results of studies and meta‐analyses when the average dosage received in the 'treatment' arms are well‐below clinical recommendations and when that dosage is probably sub therapeutic (i.e. does not reduce the AHI below diagnostic threshold).
As noted by Baker 2016, the question of whether and the extent to which OSA‐related brain structural and functional changes are reversible may be best addressed by longitudinal studies of adherent patients using within‐participants design. Unsurprisingly, improvements in cognitive ability following CPAP treatment have been found to be related to level of CPAP use. For example, Zimmerman 2006 found that memory impaired OSA patients with mean CPAP > 6 hours per night were 7.8 times more likely to experience a normalisation of memory abilities than memory‐impaired OSA patients with mean CPAP use < 2 hours per night. Moreover, although the neurocognitive domain assessed varied across studies, multiple studies have reported treatment‐related normalisation of neuropsychological functioning and neuroimaging metrics in highly‐adherent OSA patients (Canessa 2011; Castronovo 2014; Dalmases 2014; Kim 2016).
Appendix 2. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Dates searched | Frequency of search |
| CENTRAL (via the Cochrane Register of Studies (CRS)) | From inception | Monthly |
| MEDLINE (Ovid SP) ALL | 1946 onwards | Weekly |
| Embase (Ovid SP) | 1974 onwards | Weekly |
| PsycINFO (Ovid SP) | 1967 onwards | Monthly |
| CINAHL (EBSCO) | 1937 onwards | Monthly |
| AMED (EBSCO) | From inception | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IMyhill 201RG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the Cochrane Airways Trials Register
Sleep apnoea search
1. exp Sleep Apnea Syndromes/
2. (sleep$ adj3 (apnoea$ or apnoea$)).mp.
3. (hypopnoea$ or hypopnoea$).mp.
4. OSA.mp.
5. SHS.mp.
6. OSAHS.mp.
7. or/1‐6
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and the RCT filter are adapted to identify trials in other electronic databases.
Appendix 3. Search strategies and results
A. Search Strategies: Cochrane Airways Trials Register (via the Cochrane Register of Studies)
| Strategy | Details |
| #1 | SLP:MISC1 |
| #2 | MeSH DESCRIPTOR Sleep Apnea, Obstructive |
| #3 | sleep near3 (apnoea* or apnoea*) |
| #4 | (hypopnoea* or hypopnoea*) |
| #5 | (OSA OR SHS OR OSAHS:TI,AB) |
| #6 | #1 OR #2 OR #3 OR #4 OR #5 |
| #7 | MESH DESCRIPTOR Continuous Positive Airway Pressure |
| #8 | CPAP or Auto‐CPAP or APAP or NCPAP |
| #9 | (continuous* OR nasal* OR inspiratory*) NEAR "positive airway" |
| #10 | (positive* or expiratory*) NEAR (pressure*) |
| #11 | (PEEP or IPB or IPPB):ti,ab |
| #12 | #7 OR #8 OR #9 OR #10 OR #11 |
| #13 | #12 AND #5 |
| #14 | MESH DESCRIPTOR Education |
| #15 | MESH DESCRIPTOR Patient Education as Topic |
| #16 | MESH DESCRIPTOR Health Promotion EXPLODE ALL |
| #17 | MESH DESCRIPTOR Self‐Management |
| #18 | MESH DESCRIPTOR Self care |
| #19 | educat* or train* or instruct* |
| #20 | self‐manage* or self manage* |
| #21 | Self‐care* or self care* |
| #22 | support*:ti,ab |
| #23 | MESH DESCRIPTOR Behavior Therapy EXPLODE ALL |
| #24 | (behavior* or behaviour*):ti,ab,kw |
| #25 | psychotherap*:ti,ab,kw |
| #26 | MESH DESCRIPTOR Motivational Interviewing |
| #27 | MESH DESCRIPTOR Motivation EXPLODE ALL |
| #28 | motivat*:ti,ab,kw |
| #29 | cognitive*:ti,ab,kw |
| #30 | self‐efficacy:ti,ab,kw |
| #31 | MeSH DESCRIPTOR Patient Compliance Explode All |
| #32 | MeSH DESCRIPTOR Patient Acceptance of Health Care Explode All |
| #33 | adhere* or nonadhere* or non‐adhere* |
| #34 | accept* or comply or compliance or reinforce* |
| #35 | #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 |
| #36 | #35 AND #13 |
| #37 | INREGISTER |
| #38 | #36 AND #37 |
B. Search Strategies: ClinicalTrials.gov
| Field | Search Term |
| Condition | Sleep Apnoea |
| Intervention | CPAP OR continuous positive airway pressure |
| Other search terms | adherence OR compliance OR comply |
| Study type | Interventional Studies |
adherence OR compliance OR comply | Interventional Studies | Sleep Apnea | CPAP OR continuous positive airway pressure
C. Search Strategies: WHO International Clinical Trials Registry Platform
Condition sleep apnoea
Intervention CPAP
D. Search Strategies: Epistemonikos (International Systematic Review Database)
(title:(sleep apnoea OR sleep apnoea) OR abstract:(sleep apnoea OR sleep apnoea)) AND (title:(CPAP OR continuous positive airway pressure) OR abstract:(CPAP OR continuous positive airway pressure)) AND (title:(education OR behaviour OR behavior OR motivat* OR cognitiv* OR adhere* OR self‐manage* OR psychotherap*) OR abstract:(education OR behaviour OR behavior OR motivat* OR cognitiv* OR adhere* OR self‐manage* OR psychotherap*))
Limits=Systematic reviews
E. Results of main search
| Database | Years searched | Date of search | References before de‐duplication | References after de‐duplication | Comments |
| Airways Register (via the CRS) | all | 2018/07/11 | 861 | 861 | |
| Clinicaltrial.gov | all | 2018/07/11 | 162 | 147 | |
| WHO trials portal | all | 2018/07/11 | 122 | 94 | |
| Epistemonikos | all | 2018/08/29 | 32 | ‐ | Search for related systematic reviews |
| Total | 1177 | 1102 | 1074; 28 included refs; 4 awaiting classification |
F. Results of updated search
| Database | Years searched | Date of search | References before de‐duplication | References after de‐duplication |
| Airways Register (via the CRS) | July 2018‐April 2019 | 2019/04/29 | 167 | 167 |
| Clinicaltrial.gov | July 2018‐April 2019 | 2019/04/29 | 9 | 9 |
| WHO trials portal | July 2018‐April 2019 | 2019/04/29 | 7 | 6 |
| Total | 183 | 182 | ||
Data and analyses
Comparison 1. Educational intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CPAP Device Usage (hours/night) | 10 | 1128 | Mean Difference (IV, Random, 95% CI) | 0.85 [0.32, 1.39] |
| 2 Machine usage, sensitivity analysis: adherence in control group < four hours/night | 6 | 698 | Mean Difference (IV, Random, 95% CI) | 0.85 [0.06, 1.64] |
| 3 N deemed adherent (≥ four hours/night) | 7 | 1019 | Odds Ratio (M‐H, Random, 95% CI) | 2.58 [1.50, 4.44] |
| 4 Withdrawal | 9 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Epworth Sleepiness Scale ‐ Comparison of Values at Endpoint | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
1.2. Analysis.
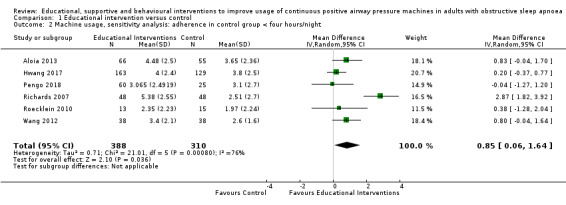
Comparison 1 Educational intervention versus control, Outcome 2 Machine usage, sensitivity analysis: adherence in control group < four hours/night.
1.4. Analysis.
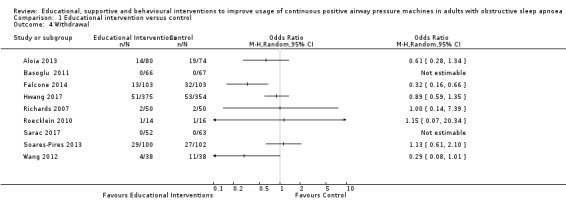
Comparison 1 Educational intervention versus control, Outcome 4 Withdrawal.
1.5. Analysis.
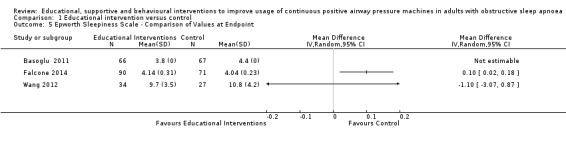
Comparison 1 Educational intervention versus control, Outcome 5 Epworth Sleepiness Scale ‐ Comparison of Values at Endpoint.
Comparison 2. Supportive intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CPAP Device Usage (hours/night) | 13 | 1426 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.36, 1.05] |
| 2 Machine usage, sensitivity analysis: adherence in control group < four hours/night | 7 | 735 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [0.57, 1.25] |
| 3 N deemed adherent (≥ four hours/night) | 2 | 376 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.08, 2.60] |
| 4 Withdrawals | 11 | 1702 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.97, 1.66] |
| 5 Epworth Sleepiness Scale ‐ Comparison Endpoint or Change from Baseline Values | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 ESS: Endpoint Scores | 6 | 700 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.59, 0.64] |
| 5.2 ESS: Change from Baseline | 5 | 470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐1.19, 0.56] |
| 6 Quality of Life: Comparison of Values at Endpoint | 7 | 683 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.01, 0.31] |
| 6.1 QoL: FOSQ ‐ Endpoint | 3 | 109 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.23, 0.53] |
| 6.2 QoL: SAQLI ‐ Endpoint | 1 | 240 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.04, 0.47] |
| 6.3 QoL: SF‐36 (PH) ‐ Endpoint | 3 | 334 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.09, 0.34] |
| 7 Quality of LIfe: Comparison of Change from Baseline Values | 3 | 294 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.01, 0.45] |
| 7.1 QoL: FOSQ ‐ Change from Baseline | 1 | 39 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.40, 0.87] |
| 7.2 QoL: SF‐36 (PH) ‐ Change from Baseline | 1 | 82 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.40, 0.47] |
| 7.3 QoL: FOSQ‐10 ‐ Change from Baseline | 1 | 173 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.00, 0.60] |
| 8 Anxiety Symptom Rating (HADS‐A) ‐ Comparison of Values at Endpoint | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Machine usage, sensitivity analysis: excluding study with opposite direction of effect (authors suggest negative effect of intervention) | 12 | 1319 | Mean Difference (IV, Random, 95% CI) | 0.74 [0.49, 0.98] |
| 10 AHI on treatment ‐ Comparison of Values at Endpoint | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Depression Symptom Rating (HADS‐D, CES‐D) ‐ Comparison of Values at Endpoint | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 HADS‐Depression | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 CES‐D | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Cost‐Effectiveness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Machine usage, sensitivity analysis: excluding participants aware of machine usage | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.6. Analysis.
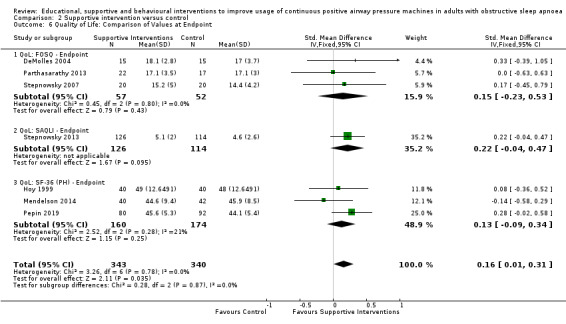
Comparison 2 Supportive intervention versus control, Outcome 6 Quality of Life: Comparison of Values at Endpoint.
2.8. Analysis.
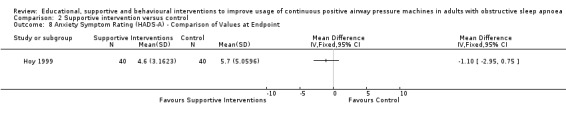
Comparison 2 Supportive intervention versus control, Outcome 8 Anxiety Symptom Rating (HADS‐A) ‐ Comparison of Values at Endpoint.
2.10. Analysis.
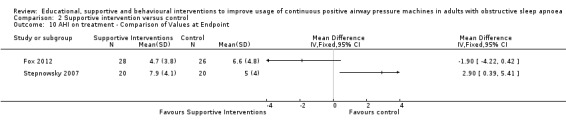
Comparison 2 Supportive intervention versus control, Outcome 10 AHI on treatment ‐ Comparison of Values at Endpoint.
2.11. Analysis.
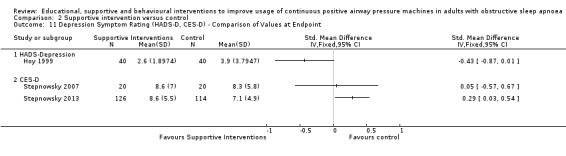
Comparison 2 Supportive intervention versus control, Outcome 11 Depression Symptom Rating (HADS‐D, CES‐D) ‐ Comparison of Values at Endpoint.
2.12. Analysis.

Comparison 2 Supportive intervention versus control, Outcome 12 Cost‐Effectiveness.
2.13. Analysis.
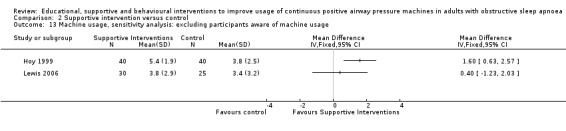
Comparison 2 Supportive intervention versus control, Outcome 13 Machine usage, sensitivity analysis: excluding participants aware of machine usage.
Comparison 3. Behavioural intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CPAP Device Usage (hours/night) | 8 | 578 | Mean Difference (IV, Fixed, 95% CI) | 1.31 [0.95, 1.66] |
| 2 CPAP Device Usage (hours/night), sensitivity analysis: adherence in control group < four hours/night | 6 | 525 | Mean Difference (IV, Fixed, 95% CI) | 1.32 [0.93, 1.72] |
| 3 N deemed adherent (≥ four hours/night) | 6 | 549 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.20, 2.41] |
| 4 Withdrawal | 10 | 939 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.44, 0.98] |
| 5 Epworth Sleepiness Scale (Endpoint scores) | 5 | 271 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐4.27, ‐0.57] |
| 6 AHI on treatment ‐ Endpoint | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐2.25, 0.35] |
| 7 Quality of Life ‐ Comparison of Values at Endpoint | 3 | 228 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.26, 0.26] |
| 7.1 QoL: FOSQ ‐ Endpoint | 2 | 200 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.26, 0.29] |
| 7.2 QoL: SF‐36 (PH) ‐ Endpoint | 1 | 28 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.82, 0.67] |
Comparison 4. Mixed (SUP/EDU/BEH) intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CPAP Device Usage (hours/night) | 11 | 4509 | Mean Difference (IV, Random, 95% CI) | 0.82 [0.20, 1.43] |
| 2 CPAP Device Usage, sensitivity analysis: adherence in control group < four hours/night | 2 | 343 | Mean Difference (IV, Random, 95% CI) | 1.77 [0.21, 3.34] |
| 3 N deemed adherent (≥ four hours/night) | 9 | 4015 | Odds Ratio (M‐H, Random, 95% CI) | 1.71 [1.08, 2.72] |
| 4 Withdrawal | 11 | 4956 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.28, 1.30] |
| 5 Quality of LIfe: Comparison of Change from Baseline Values | 2 | 3012 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.12, 0.78] |
| 5.1 QoL: FOSQ‐10 ‐ Change from Baseline | 1 | 176 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.05, 0.54] |
| 5.2 QoL: SF‐36 (PH) ‐ Change from Baseline | 1 | 2836 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.52, 0.67] |
| 6 Quality of Life: Comparison of Values at Endpoint | 4 | 3191 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.06, 0.83] |
| 6.1 QoL: FOSQ ‐ Endpoint | 1 | 177 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.19, 0.40] |
| 6.2 QoL: SF‐36 (PH) ‐ Endpoint | 3 | 3014 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [‐0.01, 1.19] |
| 7 Anxiety Symptom Rating ‐ Comparison of Values at Endpoint | 3 | 333 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.47, 0.09] |
| 7.1 DASS ‐ Anxiety | 1 | 177 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.32, 0.27] |
| 7.2 BAI | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.63, 0.34] |
| 7.3 STAI ‐ State | 1 | 91 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.92, ‐0.06] |
| 8 Depression Symptom Rating ‐ Comparison of Values at Endpoint | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 BDI | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 HADS ‐ Depression | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 DASS ‐ Depression | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Epworth Sleepiness Scale Score | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 ESS: Endpoint Scores | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 ESS: Change from Baseline | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.2. Analysis.
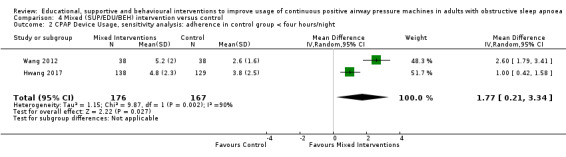
Comparison 4 Mixed (SUP/EDU/BEH) intervention versus control, Outcome 2 CPAP Device Usage, sensitivity analysis: adherence in control group < four hours/night.
4.8. Analysis.
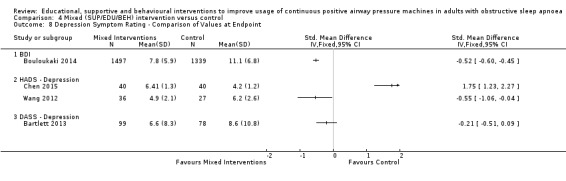
Comparison 4 Mixed (SUP/EDU/BEH) intervention versus control, Outcome 8 Depression Symptom Rating ‐ Comparison of Values at Endpoint.
4.9. Analysis.
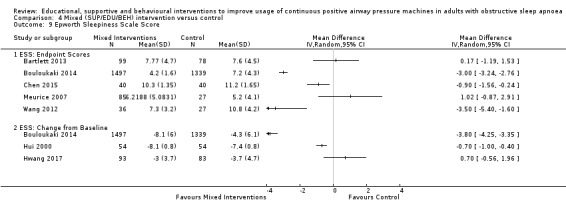
Comparison 4 Mixed (SUP/EDU/BEH) intervention versus control, Outcome 9 Epworth Sleepiness Scale Score.
Comparison 5. Post‐hoc sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 EDU: CPAP Device Usage (hours/night), original EDU study classification | 8 | 1095 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.21, 0.76] |
| 2 SUP: CPAP Device Usage (hours/night), original SUP study classification | 14 | 1534 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [0.36, 0.81] |
| 3 BEH: CPAP Device Usage (hours/night), original BEH study classification | 9 | 625 | Mean Difference (IV, Fixed, 95% CI) | 1.47 [1.12, 1.83] |
| 4 EDU: CPAP Device Usage (hours/night), exclude HIGH 'Risk of bias' studies | 4 | 642 | Mean Difference (IV, Random, 95% CI) | 0.98 [0.07, 1.89] |
| 5 SUP: CPAP Device Usage (hours/night), exclude HIGH 'Risk of bias' studies | 5 | 728 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [0.42, 1.09] |
| 6 BEH: CPAP Device Usage (hours/night), exclude HIGH 'Risk of bias' studies | 4 | 340 | Mean Difference (IV, Fixed, 95% CI) | 1.05 [0.57, 1.53] |
| 7 MIX: CPAP Device Usage (hours/night), exclude HIGH 'Risk of bias' studies | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
5.7. Analysis.
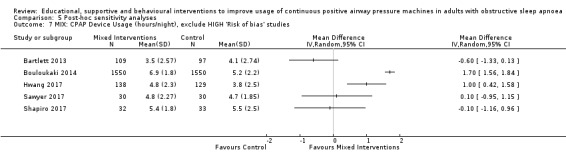
Comparison 5 Post‐hoc sensitivity analyses, Outcome 7 MIX: CPAP Device Usage (hours/night), exclude HIGH 'Risk of bias' studies.
Comparison 6. Post‐hoc subgroup analyses (exploratory).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 EDU: CPAP Device Usage (hours/night) | 10 | 1128 | Mean Difference (IV, Random, 95% CI) | 0.85 [0.32, 1.39] |
| 1.1 Intervention Duration > 4 weeks | 3 | 453 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.10, 0.77] |
| 1.2 Intervention Duration ≤ 4 weeks | 7 | 675 | Mean Difference (IV, Random, 95% CI) | 1.20 [0.39, 2.01] |
| 2 EDU: CPAP Device Usage (hours/night) | 9 | 836 | Mean Difference (IV, Random, 95% CI) | 0.98 [0.36, 1.59] |
| 2.1 Contact Episodes: > 1 | 6 | 514 | Mean Difference (IV, Random, 95% CI) | 1.20 [0.41, 2.00] |
| 2.2 Contact Episodes: 1 (single) | 3 | 322 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.06, 0.86] |
| 3 EDU: CPAP Device Usage (hours/night) | 8 | 808 | Mean Difference (IV, Random, 95% CI) | 1.04 [0.37, 1.71] |
| 3.1 Contact Time > 60 min | 3 | 293 | Mean Difference (IV, Random, 95% CI) | 1.46 [0.22, 2.71] |
| 3.2 Contact Time ≤ 60 min | 5 | 515 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.00, 1.22] |
| 4 SUP: Subgroup Analysis ‐ CPAP Device Usage (hours/night) | 13 | 1426 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.36, 1.05] |
| 4.1 Intervention Duration > 12 weeks | 4 | 530 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐0.53, 1.51] |
| 4.2 Intervention duration ≤12 weeks | 9 | 896 | Mean Difference (IV, Random, 95% CI) | 0.72 [0.43, 1.01] |
| 5 SUP: Subgroup Analysis ‐ CPAP Device Usage (hours/night) | 13 | 1426 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.36, 1.05] |
| 5.1 Intervention entailed Automated Contact Only | 4 | 513 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.51, 1.04] |
| 5.2 Intervention included Human Contact | 9 | 913 | Mean Difference (IV, Random, 95% CI) | 0.84 [0.52, 1.17] |
| 6 SUP: Subgroup Analysis ‐ CPAP Device Usage (hours/night) | 13 | 1426 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [0.41, 0.89] |
| 6.1 Scheduled, Human Interaction | 3 | 136 | Mean Difference (IV, Fixed, 95% CI) | 1.43 [0.61, 2.24] |
| 6.2 Automated and/or Ad‐hoc Human Contact only | 10 | 1290 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [0.33, 0.83] |
| 7 BEH: Subgroup Analysis ‐ CPAP Device Usage (hours/night) | 8 | 577 | Mean Difference (IV, Random, 95% CI) | 1.31 [0.95, 1.66] |
| 7.1 BEH: Intervention Duration > 4 weeks | 3 | 208 | Mean Difference (IV, Random, 95% CI) | 1.21 [0.60, 1.82] |
| 7.2 BEH: Intervention Duration ≤ 4 weeks | 5 | 369 | Mean Difference (IV, Random, 95% CI) | 1.38 [0.80, 1.95] |
| 8 BEH: Subgroup Analysis ‐ CPAP Device Usage (hours/night) | 8 | 577 | Mean Difference (IV, Random, 95% CI) | 1.31 [0.95, 1.66] |
| 8.1 BEH: Contact Episodes: > 1 | 7 | 537 | Mean Difference (IV, Random, 95% CI) | 1.35 [0.94, 1.77] |
| 8.2 BEH: Contact Episodes: 1 (single) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 1.10 [0.26, 1.94] |
| 9 BEH: Subgroup Analysis ‐ CPAP Device Usage (hours/night) | 8 | 577 | Mean Difference (IV, Random, 95% CI) | 1.31 [0.95, 1.66] |
| 9.1 BEH: Contact Time > 60 | 6 | 437 | Mean Difference (IV, Random, 95% CI) | 1.15 [0.71, 1.60] |
| 9.2 BEH: Contact Time ≤ 60 | 2 | 140 | Mean Difference (IV, Random, 95% CI) | 1.56 [0.68, 2.44] |
| 10 MIX: Subgroup Analysis ‐ CPAP Device Usage (hours/night) | 11 | 4509 | Mean Difference (IV, Random, 95% CI) | 0.82 [0.20, 1.43] |
| 10.1 Intervention Duration > 4 weeks | 8 | 4178 | Mean Difference (IV, Random, 95% CI) | 1.22 [0.60, 1.83] |
| 10.2 Intervention Duration ≤ 4 weeks | 3 | 331 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.83, 0.21] |
| 11 MIX: Subgroup Analysis ‐ CPAP Device Usage (hours/night) | 10 | 4242 | Mean Difference (IV, Random, 95% CI) | 0.79 [0.10, 1.48] |
| 11.1 Contact Episodes: > 1 | 9 | 4036 | Mean Difference (IV, Random, 95% CI) | 0.98 [0.32, 1.63] |
| 11.2 Contact Episodes: 1 (single) | 1 | 206 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.33, 0.13] |
| 12 MIX: Subgroup Analysis ‐ CPAP Device Usage (hours/night) | 10 | 4242 | Mean Difference (IV, Random, 95% CI) | 0.79 [0.10, 1.48] |
| 12.1 Contact Time > 60 min | 6 | 3751 | Mean Difference (IV, Random, 95% CI) | 1.45 [0.73, 2.16] |
| 12.2 Contact Time ≤ 60 min | 4 | 491 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.56, 0.27] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aloia 2001.
| Methods | Randomised parallel‐group trial. | |
| Participants |
N = 12 existing patients at investigator sleep centre with OSA. Participants had received prior treatment with CPAP Inclusion criteria: > 55 years of age, RDI (AHI): > 10, Mini Mental Status Examination: > 25 Exclusion criteria: other ICSD, other treatment for apnoea, claustrophobia Baseline characteristics: mean age: 63.4, AHI: 43.5, Desaturation: 77.05 ± 9.47. Baseline characteristics not reported: gender, BMI, ESS. Country: USA |
|
| Interventions | Participants were randomised into experimental intervention (n = 6) or control (n = 6). Intervention: two sessions. Session 1: review of participants' sleep data; symptoms; review of performance of cognitive tests; review of importance of treatment; review of PSG and CPAP; discussion of advantages and disadvantages of treatment; development of goals for therapy. Session 2: examination of compliance data for week one; discussion of noticeable changes with treatment; discussion of changes not apparent (hypertension/cardiac problems); troubleshooting discomfort; discussion of realistic aims of treatment; review of treatment goals Control: two sessions: general discussion of sleep architecture and opinions on sleep clinic Study duration: 12 weeks |
|
| Outcomes |
|
|
| Notes | * Indicates primary outcome analysed in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by 'urns', stratification by age, RDI, nadir O2 pretreatment, vigilance |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not done for treatment group assignment 'None of the participants were told that their CPAP machines were measuring their compliance via internal microprocessors' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | High risk | Authors describe randomisation procedures in METHODS: "...participants were randomised into one of two groups (experimental or control) based on their age, RDI, and nadir oxygen level pretreatment. This type of randomisation involves placing participants into groups based on the current group averages for variables to be randomised. For example, if the average age of group 1 is 50 years and the average age of group 2 is 60 years, the new 40‐year‐old subject would be placed in group 2 to approximate the first group's average age. Accordingly, the two groups were not significantly different on age, education, RDI, or nighttime oxygen saturation." Based upon cited reference (Stout et al, 1994) for these procedures, this suggests a form of urn randomisation was used. Urn randomisation may be adequate (Cochrane Risk of bias 2 reference manual) and even recommended to ensure balance with small sample sizes, where major imbalance could occur with higher probability. (Hedden, et al, 2006 Randomisation in substance abuse clinical trials; Wei & Lachin, Properties of the urn randomisation in clinical trials. Controlled Clinical Trials 1998;9(4):345‐64). No reference to allocation concealment method. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. Authors report, "Machines were switched unexpectedly in the middle of the study for three participants for reasons unrelated to treatment. Calculating cumulative run time at the 12‐week follow‐up instead of night‐by‐night figures accommodated this event. participants' cumulative data were then divided by the number of nights with the machine to obtain average daily run time." This deviation was likely unrelated to experimental context. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Information not available |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Unclear risk | Outcome measured using objective CPAP usage data. Insufficient information. All participants received the same CPAP device at the start of the trial. However, authors report, "Machines were switched unexpectedly in the middle of the study for three participants for reasons unrelated to treatment. Calculating cumulative run time at the 12‐week follow‐up instead of night‐by‐night figures accommodated this event. Participants' cumulative data were then divided by the number of nights with the machine to obtain average daily run time." The group assignment of these three participants was not reported and the meaning of 'machines were switched' is unclear. If this implies a switch to a different device make or model, it is possible that outcome measurement could have differed between the intervention group. Given the small N and depending upon the distribution of the 'switches' between groups, this could have impacted effect size estimates. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. Methods section indicates multiple outcome time points (for primary adherence outcome) were planned. Results section reports each planned time point. No threshold‐defined adherence outcomes were specified in Methods; one was reported in results. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Aloia 2013.
| Methods | Randomised parallel‐group trial. | |
| Participants |
N= 227 with OSA. Inclusion criteria: age 25‐85 years, moderate to severe OSA (AHI > 15) by full in‐laboratory overnight polysomnography, naive to PAP therapy. Exclusion criteria: Diagnosis by split‐night PSG; evidence of severe neurological condition or unstable psychiatric illness; sleep disorder other than OSA (including primary central sleep apnoea), CHF, ESRD. Baseline characteristics: 34% female. Mean age 50.2 (±11.1). Mean AHI 46.7. Mean ESS 12.1. Mean BMI 35.3. Country: USA |
|
| Interventions | Participants were urn randomised in a 1:1:1 ratio into one of three groups ‐ standard care (n = 74), education (n = 80) and motivational enhancement therapy (n =73) ‐ balancing for age, sex, education, apnoea severity, and ESS score. Individuals in the MET and ED groups each received two, 45‐minute, face‐to‐face individual counselling sessions by a trained nurse 1 week (7 ± 2 days) and 2 weeks (14 ± 2 days) after initiating PAP treatment. Intervention sessions were delivered after 1 week of PAP use. One additional booster phone call was made to each participant in the motivational enhancement therapy and education groups at week 3 of PAP use. MET: manualised MET intervention aimed at helping patients resolve their ambivalence regarding consistent PAP use, tailored based on assessment of each participants readiness for change. ED: education regarding pathophysiology of apnoea, its medical and behavioural consequences, and the benefits of treatment; presented in standardised formats, with no tailoring to participant readiness. SC: provided to all participants, consisted of standard clinical care delivered by the authors' sleep disorders centre. Study duration: 12 months |
|
| Outcomes |
Adherence was measured nightly during the course of the year‐long study. Participant average adherence from the beginning of the experiment up to 1, 2, 3, 6 and 12 months were used in analyses, i.e. cumulative mean responses were used). Decisional balance measure consists of both pro items, which assess the benefits of engaging in a particular behaviour, and con items, which assess the costs to the patient of engaging in PAP adherence. A five‐point Likert scale was used to rate each item, with 1 being "not important at all" and 5 being "extremely important." The self‐efficacy scale was constructed using assess the extent to which patients believed that they could do the required tasks. Decisional balance and self‐efficacy measurements were taken concurrent with the 3‐, 6‐, and 12‐month PAP adherence measurements |
|
| Notes | Trialists included two intervention arms, one educational and one behavioural. MET vs. Control included in behavioural meta‐analysis. ED vs. control included in Educational meta‐analysis. * Indicates primary outcome analysed in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...participants were randomised into one of two groups (experimental or control) based on their age, RDI, and nadir oxygen level pretreatment" |
| Allocation concealment (selection bias) | Unclear risk | No reference to allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were available for all, or nearly all, participants randomised. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but it is clear that the published reports include all expected outcomes. |
| Other bias | Low risk | Although there was an imbalance in age, it is unlikely to impact the outcome. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | "Urn randomisation (Wei & Lachin, 1988) is conducted by tossing a (possibly biased) coin each time a patient is to be allocated. Heads indicates active treatment, and tails indicates control." (Berger & Christophi, 2003). Trialists provided no information regarding random component used in sequence generation or on how sequence concealment was achieved. Authors report "Participants were urn randomised in a 1:1:1 ratio into one of three groups (MET, ED, or SC) balancing for age, sex, education, apnoea severity, and Epworth Sleepiness Scale score." Baseline characteristics for randomised participants did show imbalance (P < 0.005). However, sample sizes may have made balancing across multiple factors difficult to achieve. Moreover, the difference does not appear to be large enough for the resulting confounding to bias the intervention effect estimate, particularly as the observed differential age distribution is unlikely to be clinically‐significant. That is, the mean ages across groups were 52.4 (SC), 47.0 (ED) and 51.7 (MET). Clinically, a large difference in compliance would not be reliably predicted based on differences in adherence behaviours between participants aged 47 and those aged 52.4. There is some evidence that age < 49 may predict worse (medication) adherence across disorders (Rolnick 2013), but confounding on the basis of age in this direction would have resulted in lower adherence in the ED group; the results of this study found nominally higher adherence in the ED group. Most studies examining CPAP adherence specifically, focus on differences between adults and 'older adults,' usually defining the latter as > 60 years. Thus, these studies are not relevant to the age differentials observed between groups in this study. Moreover, the age‐related differences in CPAP adherence found in those studies varied in direction by study ‐ some finding higher and others finding lower adherence among older adults. (See Sawyer 2011, Sleep Med Rev for review). |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Neither participants nor those delivering interventions were blinded to intervention assignment. Physicians and other healthcare providers (non‐research staff) were blind to enrolment. No deviations documented; none suspected based upon review. Only observed deviations from planned intervention is non‐compliance with behavioural intervention which is typical of routine care, so unrelated to the experimental context. Likely mITT. Protocol deviation was unlikely to have contributed to biased effect estimates as it was balanced across intervention arms and likely unrelated to outcome. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Unclear risk | The authors did not use analysis methods that correct for bias nor did they perform sensitivity analyses showing that results are little changed under a range of plausible assumptions about relationship between missingness and outcome true value. Reasons for missing outcome were not documented in the study report (or NCT listing), so could not be assessed for potential relation to outcome. The authors report that they found no differences in dropout rates between groups. The authors also report that they compared dropouts and completers on demographic and severity variables and found no between‐group differences. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Low risk | Primary outcome measures submitted to clinicaltrials.gov (NCT00623246) included adherence to CPAP measured at months 3, 6, 12. These time points as well as two additional time points (1 and 2 months) were included in published report. All planned time points results were reported. Multiple analyses (e.g. variable adherence 'thresholds') not conducted. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Bakker 2016.
| Methods | Open‐label, parallel‐arm, RCT. | |
| Participants |
N = 83 participants with OSA Inclusion criteria: AHI 4%, ≥ 10 or AHI 3%, ≥ 15; 45 to 75 years with established CVD or cardiometabolic disease (established coronary artery disease (≥ 70% stenosis in at least one major coronary artery), prior myocardial infarction, coronary artery revascularisation procedure, Ischaemic stroke, or diabetes) OR 55 to 75 years with at least three CVD risk factors (male sex, BMI ≥ 30, hypertension, dyslipidaemia, and ≥ 10 pack‐years of smoking). Exclusion criteria: cardiovascular event < 4 months before enrolment, prior CPAP, ESS > 14 of 24, drowsy driving within 2 years, commercial driving, or an uncontrolled medical condition (including CSA, heart failure, uncontrolled hypertension, severe hypoxaemia, anaemia, and renal insufficiency). Baseline characteristics: 33% female. Mean age 63.8 (NR). Mean AHI 22.8. Mean ESS NR. Mean BMI 31.1. Country: USA |
|
| Interventions | Eligible participants entered a run‐in phase before randomisation, consisting of 14 days wearing a nasal CPAP mask during sleep (without a CPAP device). Participants who reported using the mask during the majority of the run‐in and who were willing to continue using the mask were eligible for randomisation. Randomisation took place in a 1:1:1:1 ratio with a block size of 4, based on three stratification factors: diagnostic study (full night or split night with titration), site, CVD status (established or risk factors) to one of four study arms (two control conditions, two treatment conditions): conservative medical therapy (n = 44), sham CPAP (n = 42), active CPAP (n = 42), or active CPAP +ME (n = 41). Bakker 2016 reported only the active CPAP and CPAP + ME arms. Intervention (Active CPAP + ME): overall goal of each ME session was to resolve the participants' ambivalence toward establishing consistent CPAP usage patterns and increase their confidence toward using CPAP regularly. Each participant was encourage to set concrete goals regarding their future CPAP use and identify rewards that they could provide themselves when those goals were achieved. ME was delivered during 1‐hour in‐person sessions at baseline and week 1, which included an educational video, and during phone calls of 10 to 30 minutes with the same psychologist at weeks 3, 4, 8, 12, 20, and 32. Control (Active CPAP): CPAP Study duration: 12 months. |
|
| Outcomes |
|
|
| Notes | * Indicates primary outcome analysed in this Review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization took place in a 1:1:1:1 ratio with a block size of 4, based on three stratification factors: diagnostic study (full night or split night with titration), site (BWH, BIDMC, or Joslin), CVD status (established or risk factors). Randomization was performed using a data‐entry system linked to an off‐site server holding the sequences." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to determine sufficiency of sequence concealment: Sequences were concealed at off‐site location. Block sizes were fixed, so last assignment to each block could be predicted, but only likely to occur if investigators were aware of block sizes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were available for all, or nearly all, participants randomised. |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but thorough analysis of information presented in study's NCT archive does not suggest that outcomes of interest were chosen after data analysis already commenced. |
| Other bias | Low risk | No baseline imbalances or reported deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Authors report, "Analyses in this paper compare the active CPAP and CPAP + ME arms only. Randomization took place in a 1:1:1:1 ratio with a block size of 4, based on three stratification factors: diagnostic study (full night or split night with titration), site (BWH, BIDMC, or Joslin), CVD status (established or risk factors). Randomization was performed using a data‐entry system linked to an off‐site server holding the sequences. Participants randomly assigned to a CPAP group immediately underwent secondary randomisation to use a device by one of two manufacturers (Philips Respironics or ResMed), using a randomisation sequence with a block size of 2." From Protocol (Yaggi 2016): 255 patients with TIA or stroke are randomly assigned using a 1:1:1 (control:standard PAP: enhanced PAP) randomisation scheme to either usual care or a home‐based diagnosis and treatment approach that includes ambulatory polysomnography and initiation of PAP for patients with sleep apnoea (Figure 1). Control patients could undergo diagnosis and treatment of sleep apnoea if suspected as part of usual clinical care. The randomisation is stratified by centre (Connecticut or Indiana) and neurologic event type (TIA or stroke)...."
Random component used for sequence generation not explicitly described. Insufficient information to determine sufficiency of sequence concealment: Sequences were concealed at off‐site location. Block sizes were fixed, so last assignment to each block could be predicted, but only likely to occur if investigators were aware of block sizes. Authors provide a tabular summary of baseline characteristics for participants randomised to CPAP+ME and CPAP‐only intervention arms, and report in text: "The two groups were comparable at baseline in terms of age, sex, anthropometrics, race or ethnicity, and cardiovascular risk factors (Table 5)." |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Yaggi 2016 report that participants were unblinded. (p. 5) Deviation from protocol documented by authors: The original trial design included follow‐up for 12 months; however, participants randomly assigned after January 2013 were restricted to 6 months of follow‐up. Our primary analysis of CPAP adherence therefore took place for 6 months, rather than 12." This deviation is unlikely to have affected the outcome. Authors report, "All analyses were intention‐to‐treat." Additionally, all outcome measures appear to derive from all randomised participants in the two relevant treatment arms. Protocol deviation was unlikely to have contributed to biased effect estimates as it was balanced across intervention arms and likely unrelated to outcome. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Six‐month outcome data available for nearly all randomised participants. In addition to having primary outcome (CPAP usage) data for nearly all participants, authors documented reasons for missing adherence data and conducted sensitivity analyses, determining that the finding of no differences in adherence between groups was robust when a value of zero was assigned to missing usage data and when examining complete data only. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Published report notes, "All participants were provided with either a REMstar
Pro CPAP (Respironics Inc. Murrysville PA) device with a data‐storage Smart‐Card, or a Sandman Goodnight 420 Series Auto HH (Covidien, Mansfield, MA) which allowed usage data to be directly downloaded from the machine or via a memory key. Reviewers contacted authors to request information as to the distribution of CPAP device make across intervention arms. Authors responded (email) reporting, "Our CPAP machines were provided for the study by Respironics – REMstar Auto with C Flex or REMstar Pro M Series." Thus, the information provided by authors in personal correspondence suggests that no participants received the Sandman Goodnight 420 Series Auto HH and, therefore, that outcome measurement should not have differed between intervention groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk |
NCT01261390: Original primary outcome measures of BestAir trial (submitted 15 Dec 2010) was "Effectiveness of continuous positive airway pressure therapy on cardiovascular disease, using mean 24 hour systolic blood pressure as the trial's primary endpoint. [ Time Frame: 3 years ]". Current primary outcome measures (submitted 18 Apr 2017) include those of interest in this review: "Difference in CPAP Adherence by Active Treatment Arm [ Time Frame: 6‐months ] Original secondary outcome measure (submitted 15dec2010): "Recruitment and retention rates of patients with moderate to severe obstructive sleep apnoea and cardiovascular disease risk factors or established CVD participating in a controlled trial. [ Time Frame: 3 years ]"
"Adherence to CPAP therapy was tracked remotely by modem transmission. Outcome reported is mean hours of PAP use per night at the 6‐month time point. Comparison is between those with and without assignment to Motivational Enhancement as part of treatment randomisation." Current secondary outcome measures of relevance to our review (submitted 18 Apr 2017): Change in ESS, SAQLI, SF‐36, PHQ‐8 [6 & 12 months.
Though it appears that CPAP adherence was a target of the intervention from the outset (i.e. it is repeatedly mentioned as a focus of attention in description of the active treatment arms), it does not appear to have been an outcome of interest at the time of design. Rather, as described in the protocol (described in Yaggi 2016), adherence was a primary process measure (and one likely to impact the primary physiological outcome of interest). Study primary completion date (March 2014, final collection date for primary outcome measure) occurred before current primary/secondary outcome measures posted to clinicaltrials.gov. However, the time stamps provided in NCT entry are merely the date that these outcomes were entered into the electronic system and likely do not represent the date that decisions about additional outcome measures were made. Thus, it is possible that all primary/secondary outcomes were finalised before unblinded outcome data were available for analysis. Decision to change primary CPAP adherence outcome from 12‐month to 6‐month endpoint likely a consequence of a change in design/follow‐up necessitated by factors unrelated to the outcome that required follow‐up to shorten for participants enrolled after January, 2013. Moreover, for those participants with outcome data at 12 months, the results were similar. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Bartlett 2013.
| Methods | Randomised parallel‐group trial. | |
| Participants |
N = 206 participants with moderate‐severe OSA referred to CPAP therapy. Inclusion criteria: none reported other than moderate‐severe OSA. Exclusion criteria: unable to understand fluent English, any previous use of CPAP. Baseline characteristics: 32% female. Mean age 48.1 (±13.2). Mean AHI 34.9. Mean ESS 11.9. Mean BMI 30.4. Country: Australia |
|
| Interventions | Prior to recruitment, a randomisation sequence by group using random permuted blocks with a 1:1 allocation ratio to control arm, Social Interaction (n = 97) or intervention arm, Social Cognitive Therapy (n = 109). Social Cognitive Therapy: intervention was based on social cognitive theory factors, including perceived self‐efficacy, outcome expectations, and social support. Participants were encouraged to list goals, given slide presentations to discourage unhelpful thoughts of CPAP side effects, taught relaxation strategies, and given additional booklets containing information about sleep OSA/CPAP, and general health. Social interaction: a basic social intervention was given to ensure that equal time was spent with all study participants; SI group was shown a 15‐minute video that followed a patient's journey from their baseline diagnostic sleep study to being diagnosed with OSA and undergoing a CPAP titration study. Study duration: 6 months |
|
| Outcomes |
CPAP usage was assessed at 7 nights, then 1, 3, and 6 months. Though these endpoints were reported as intended secondary outcomes, only 6‐month endpoint was presented in published report. The two primary outcomes were adherence, usage ≥ 4 hours per night at 6 months, and uptake of CPAP (measured through the SCT questionnaire). Questionnaires, including ESS, DASS, FSS, PSQI, and FOSQ, were also administered at baseline, 1 month, and 6 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Prior to recruitment, a randomisation sequence by group using random permuted blocks with a 1:1 allocation ratio to SI and SCT interventions was generated by an independent investigator.... the psychologist responsible for the interventions opened a sequentially numbered, opaque, sealed envelope containing the randomisation allocation." |
| Allocation concealment (selection bias) | Low risk | As per trial registry entry, ACTRN12607000424404: "Allocation was concealed using sealed opaque envelopes which were opened following participant consent and prior to treatment as usual session." |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 19/97 and 10/109 patients in the SI and CBT groups withdrew from the study at 6 months, respectively. Number of withdrawals is unbalanced between groups and is >10% in one group. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT entry) and all of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | No baseline imbalances or reported deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Low risk | Authors report, "Prior to recruitment, a randomisation sequence by group using random permuted blocks with a 1:1 allocation ratio to SI and SCT interventions was generated by an independent investigator.... the psychologist responsible for the interventions opened a sequentially numbered, opaque, sealed envelope containing the randomisation allocation."
As per trial registry entry, ACTRN12607000424404: "Allocation was concealed using sealed opaque envelopes which were opened following participant consent and prior to treatment as usual session. Following treatment as usual for CPAP participants were randomised to CBT or Social Reciprocity (SR). Sealed opaque envelopes containing treatment allocation were opened following completion of treatment as usual." Key baseline characteristics (age, gender, BMI, AHI) were reported for all randomised participants and these differences were insignificant (P values reported), except for AHI (P = 0.02), with AHI in control arm higher than that in intervention arm. Authors report a minimum of 16 (but table suggests as many as 20) comparisons of baseline characteristics, and two were found to be statistically significant at a 5% level. This suggests a slight excess in the number of baseline characteristics expected to have chance differences. However, the two characteristics found to differ significantly (AHI, arousal index) are expected to be strongly correlated. Moreover, the P values were nominally‐significant, suggesting the between‐group differences in baseline characteristics are possibly due to chance. Moreover, the between‐group difference in mean AHI is probably insufficient for the resulting confounding to bias the intervention effect estimate. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Authors report, "...Participants were then presented with the SCT or SI protocol. Staff administering the SCT or SI were not blinded to the intervention, but did not have ongoing clinical contact with the patients. Other staff members interacting with the patients in subsequent visits were blinded to group allocation." No deviations documented; none suspected based upon review. ITT. Authors report, " The analysis was by intention to treat. Participants who withdrew from the study were asked to attend the 6‐month follow‐up visit to collect outcome data. In cases where CPAP usage data from device download were missing, usage was imputed to be zero hours/night if it was confirmed with the participant that had they had refused or abandoned CPAP therapy, or else missing values were imputed by carrying forward the last observation. Because this strategy might lead to an overestimate of CPAP usage, sensitivity analyses were conducted, specifically by imputing zero usage to all missing data, or confining analysis to cases with complete data. Predictors of CPAP adherence at 6 months were explored by logistic regression, and the effect of the SCT intervention was also evaluated, adjusting for significant predictors of adherence or variables found to be different between the intervention groups at baseline. P values < 0.05 were considered significant." |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | 9 of 97 (9.2%) and 5 of 109 (4.6%) of participants in the control and intervention arms, respectively, had no outcome data at primary endpoint and had their previous adherence data carried forward for outcome measures. In addition to having primary outcome (CPAP usage) data for nearly all participants, authors documented reasons for missing adherence data and conducted sensitivity analyses, determining that the finding of no differences in adherence between groups was robust when a value of zero was assigned to missing usage data and when examining complete data only. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Published report notes, "All participants were provided with either a REMstar Pro CPAP (Respironics Inc., Murrysville PA) device with a data‐storage Smart‐Card, or a Sandman Goodnight 420 Series Auto HH (Covidien, Mansfield, MA) which allowed usage data to be directly downloaded from the machine or via a memory key." Reviewers contacted authors to request information as to the distribution of CPAP device make across intervention arms. Authors responded (email) reporting, "Our CPAP machines were provided for the study by Respironics – REMstar Auto with C Flex or REMstar Pro M Series." Thus, the information provided by authors in personal correspondence suggests that no participants received the Sandman Goodnight 420 Series Auto HH and, therefore, that outcome measurement should not have differed between intervention groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Low risk | ACTRN12607000424404 (https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82216&showHistory=true&isReview=true)
Trial registered 22 Aug 2007 and all planned outcomes submitted at that time.
Updated on 9 August 2012 4:38:27 PM to indicate that data collection is now complete.
Trial information (ACTRN12607000424404) and Methods section of published report indicates that multiple time points planned; each planned outcome reported. There were multiple analyses of CPAP adherence outcomes planned and all were reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Low risk | ‐ |
Basoglu 2011.
| Methods | Randomised, parallel‐group study | |
| Participants |
N = 133 newly diagnosed moderate‐to‐severe OSAS patients Inclusion criteria: newly diagnosed, moderate to severe OSA, CPAP‐naive Exclusion criteria: use of sedatives, drug abuse, cardiac co‐morbidities, COPD, other sleep disorders Baseline characteristics, by group: Intervention group: age: 53.7, Male sex: 82%, AHI 61, ESS: 10.3, BMI: 33.2. Control group: age: 54.4, Male sex: 70%, AHI: 57.4, ESS: 12.4, BMI: 33 Country: Turkey |
|
| Interventions | Participants were randomised into video education intervention (n = 66) or control (n = 67). Intervention: 10‐minute videotape on OSA, its consequences and CPAP therapy. In addition, routine information on diagnosis and treatment of OSA given by physician Control: standard information on OSA and CPAP therapy given by the same physician Study duration: 24 weeks |
|
| Outcomes |
|
|
| Notes | Unpublished information on study design and outcomes obtained from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by a set of numbers prepared and randomly assigned by a clinician not involved in the study |
| Allocation concealment (selection bias) | Low risk | Randomisation by a third party |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The primary investigator and the statistician were blinded to the study group assignment. Participants were aware of machine usage monitoring. Given the nature of the intervention, it is unlikely that participant blinding was achieved |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study, and no data were missing |
| Selective reporting (reporting bias) | Unclear risk | No Information. |
| Other bias | Unclear risk | No Information. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within METHODS: "The study used a randomised two‐group design. Out of 133 patients, 66 were informed about OSAS and CPAP therapy by the same doctor and also received visual education by videotape (video group), whereas only information was given to 67 of them (control group). All the patients in the video group received video education after CPAP titration, and the duration between video education and initiation of CPAP treatment was approximately 1 week." No reference to random component or allocation concealment method. Key baseline characteristics (age, gender, BMI, AHI) were reported for all randomised participants and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. Authors report "There was no dropout during the follow‐up period as the patients were called up in case of missing an appointment." Additionally, results are reported for all randomised participants. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | High risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the METHODS section of the publication. No information as to whether analysis plan was finalized before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned; each planned outcome reported. Methods indicate one threshold definition. However, the decision to use only a threshold definition, and not present average usage upon which that threshold was calculated, is inconsistent with standard reporting practices in the field and suggests that the numerical result being assessed is likely to have been selected based on the results. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Bouloukaki 2014.
| Methods | Randomised, parallel‐group study. | |
| Participants |
N= 3100 patients with newly diagnosed sleep apnoea randomised to either the standard group (usual follow‐up care) or the intensive group (additional visits, telephone calls, and education). Inclusion criteria: newly diagnosed with OSAHS by PSG, moderate‐to‐severe OSAHS, no history of previous CPAP therapy, and above‐elementary school education. Exclusion criteria: refusal to participate, refusal of CPAP therapy, CSA syndromes, obesity hypoventilation syndrome, restrictive pulmonary and restrictive chest wall diseases, severe CHF, a history of life‐threatening arrhythmias, severe cardiomyopathy, LTOT, family or personal history of mental illness, drug or alcohol abuse, severe cognitive impairment, concurrent oncological diseases, and a history of narcolepsy or restless legs syndrome. Baseline characteristics: 25% female. Mean age 55.6 (±10.2). Mean AHI 52. Mean ESS 12.1. Mean BMI 37.8. Country: Greece |
|
| Interventions | Eligible patients (n = 3100) were randomly assigned in a 1:1 ratio to receive either the standard intervention (n = 1550), of usual follow‐up care, or the intensive intervention (n = 1550), with augmented follow‐up care based on additional appointments at the CPAP clinic, telephone calls and education. Intensive intervention: patients received the same features as standard group, with the addition of follow‐up visits involving patients' partners or family. All patients attended a 15‐minute video education session cover OSAHS‐related topics, including the syndrome itself, treatment options, and the benefits of adherence to therapy. This was followed by a 10‐to 15‐minute lecture used to reinforce key concepts. During the first week of CPAP set‐up, patients were contacted by the nurse, on the second and seventh day, via telephone in order to discuss any concerns they might have regarding air pressure, mask fitting, leaks and other issues as they arose. During the first month of treatment, patients were instructed to keep a sleep diary, and were reviewed by a sleep specialist on the 15th and 30th day of treatment. Standard care: patients were reviewed in the outpatient sleep clinic at 1‐month, at 3‐month intervals during the first years, and every 6 months afterwards. During these appointments, a clinical assessment was made and patients were further encouraged to use the device. If there were doubts about compliance, the referring physician made personal contact with the patient in order to resolve barriers to adequate compliance. Study duration: 2 years |
|
| Outcomes |
Chronological data were obtained from the CPAP machine at each follow‐up appointment. Self‐reported number of nights per week and hours per night were obtained for comparison against data obtained from CPAP machine. Regular CPAP compliance was defined as using the therapy for an average of 4 hours per night on at least 70% of the nights.The estimated costs of each intervention were calculated and compared between groups. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients (n = 3100) were randomly assigned in a 1:1 ratio to receive either the standard intervention (n = 1550), of usual follow‐up care, or the intensive intervention (n = 1550), with augmented follow‐up care based on additional appointments at the CPAP clinic, telephone calls and education. Randomisation was performed using a computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | No reference to allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1349/1550 and 1501/1550 in the standard and intensive groups (respectively) either discontinued the intervention or were "lost to follow‐up". Considerably more people dropped out of the standard intervention group (therefore missing outcome data are unbalanced in numbers across intervention groups). This would not affect adherence data (which can be predicted to be 0 for those who "discontinued intervention"), but would affect subjective outcomes. |
| Selective reporting (reporting bias) | Unclear risk | No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. |
| Other bias | Low risk | No baseline imbalances or reported deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Authors report, "Eligible patients (n=3100) were randomly assigned in a 1:1 ratio to receive either the standard intervention (n=1550), of usual follow‐up care, or the intensive intervention (n=1550), with augmented follow‐up care based on additional appointments at the CPAP clinic, telephone calls and education. Randomisation was performed using a computer‐generated list of random numbers."
No reference to allocation concealment method. Key baseline characteristics (age, gender, BMI, AHI) were reported and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Authors report, "Patients were blinded to the group to which they were allocated and were followed for a minimum of 2 years." Methods for blinding were not described.
Authors specify only that the PSG scorer was blinded to 'the origin of the data." No deviations documented; none suspected based upon review. AUthors report, "Data were normally distributed. Numerical variables are presented as mean SD. Intention‐to‐treat analysis was carried out, in which all patients receiving the allocated interventions were included in the analysis." Authors do not report on missing data or how missing endpoint values were accounted for (e.g. LOCF, imputation, substitution of 0 hours use). |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Authors report, "155 of the 3100 patients, 124 receiving standard support and 31 intensive support, were lost to follow‐up: 77 (4.9%) patients in the standard group and 18 (1.1%) in the intensive group had stopped using CPAP, and 10 patients in the standard group and four in the intensive support group died after randomisation (fig. 1). All 3100 patients were included in the final analysis." Authors do not report on missing data, so it is unclear if data were missing for those who did not complete the study and, if so how these missing endpoint values were accounted for (e.g. LOCF, imputation, substitution of 0 hours use). |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Unclear risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring. Unable to confirm with study author that distribution of CPAP device makes did not differ between intervention arms. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | High risk | ClinicalTrials.gov entry (NCT02016339) indicates that the original primary outcome measure was the same as the current primary outcome measure, "Effect of intensive intervention on CPAP adherence [ Time Frame: 24 months ]" (submitted 13 Dec 2013). Final data collection date for primary outcome was June 2013. NCT outcome is the same as that presented in published report. No information as to whether analysis plan was finalized before unblinded outcome data were available for analysis. Methods section (but not NCT entry) indicates that multiple analyses of CPAP adherence outcomes were planned and all were reported in Results. Though the authors report 24‐month outcomes as specified in NCT trial listing, their choice of definitions for CPAP usage rates is atypical. Specifically, authors define the CPAP usage/night outcome as "Hours per night, on nights CPAP was used." This definition of the CPAP usage/adherence outcome was not pre‐specified (in NCT entry) and may substantially overestimate CPAP usage (in both groups) because all nights not used are excluded from numerator and denominator. Since mean use per day was likely also calculated, the decision to report mean use per effective day suggests that the numerical result being assessed was selected on the basis of the results from multiple outcome measurements. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Chen 2015.
| Methods | Randomised, parallel‐group study. | |
| Participants |
N = 85 participants with new SAHS diagnosis. Inclusion criteria: AHI >15, daytime sleepiness, two major symptoms of the syndrome, lived within 100 miles from Zhejiang. Exclusion criteria: previously received CPAP therapy, suffering with COPD, asthma, or neurological problems. Baseline characteristics: 38.3% female. Mean age 50.4 (NR). Mean AHI 54.5. Mean ESS 13. Mean BMI 32.5. Country: China |
|
| Interventions | 85 participants were randomised to nurse‐led intensive vs standard support, of which 5 refused to participate (group allocation of refusals not reported), resulting in n = 40 receiving intervention and n = 40 receiving control condition. Intervention: hospital health education, consisting of pre‐treatment 30‐minute educational video that explained the pathogen, mechanism, risks, benefit, and treatment methods for SAHS; personalised guidance from a nurse; and an SAHS Health education Manual. In addition, several patient self‐management interventions were delivered including: 15‐minute interview with nurse for troubleshooting within 5 days of receiving CPAP treatment, nurse home visits after CPAP treatment was initiated, healthy lifestyle (diet, exercise) guidance, and a psychological intervention, informing patients of the importance of maintaining a good mental state for disease rehabilitation, and teaching the patients methods and techniques on how to respond to anxiety and depression. Finally, each participant in the intervention arm received a ˜30‐minute consultation with sleep physician within 1 month of CPAP initiation. Control: ˜30‐minute consultation with sleep physician at 1, 3, 6 and 12 months. Study duration: 12 months. |
|
| Outcomes |
The data regarding the usage of CPAP by the patients were recorded and handled with professional software) at each clinical visit. All participants underwent a daytime function testing at the beginning and after 12 months of the initiation of CPAP treatment. Function testing included the following steps: an in‐house questionnaire was given to assess the severity of sleep apnoea symptoms. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "This study was designed as a randomised, single‐blinded, prospective trial of nurse‐led intensive vs standard support.... The included patients were randomised into two groups via a predetermined balanced block which was generated by tossing a coin: standard support group and intensive support group." |
| Allocation concealment (selection bias) | Unclear risk | No reference to block sizes to or other allocation concealment methods. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. Study says that "patients were blinded to the allocation and did not know to which group they were assigned", but did not explain how this was performed or maintained. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data available for 80 of 85 (94%) of randomised participants. However, it is not clear to which group these 5 missing participants belonged to ‐ there is the possibility that all 5 came from one group, which would results in 12.5% (5/40) withdrawals. This would not be critical for measuring CPAP adherence (as it is objective and can be predicted to be 0 for those who refuse to participate), but it would be critical for subjective outcomes. |
| Selective reporting (reporting bias) | High risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were not consistent with the plan specified in the Methods section of the publication. |
| Other bias | Low risk | No baseline imbalances or reported deviations from intended intervention. Although there is the issue of the 5 missing participants, this is already covered in the "Incomplete Outcome Data" domain (do not want to double count). |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Regarding the randomisation procedures, authors report: "This study was designed as a randomised, single‐blinded, prospective trial of nurse‐led intensive vs standard support.... The included patients were randomised into two groups via a predetermined balanced block which was generated by tossing a coin: standard support group and intensive support group." No reference to block sizes to or other allocation concealment methods. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Unclear risk | Authors report: "Patients were blinded to the allocation and did not know to which group they were assigned." No information was provided as to how blinding was performed/maintained. After randomisation, n = 5 participants 'refused to participate.' No further information was provided, including: group allocation of dropouts, reasons for refusal. This suggests the possibility that the deviation arose because of experimental context/expectation of differences between groups (e.g. disappointment about assignment). mITT used. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Data available for 80 of 85 (94%) of participants. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were not consistent with the plan specified in the Methods section of the publication. Description of intended outcome measurements (time points) was very limited: "All participants underwent a daytime function testing at the beginning and after 12 months of the initiation of CPAP treatment. Function testing included the following steps: an in‐house questionnaire was given to assess the severity of sleep apnoea symptoms; Epworth and Stanford sleepiness scales were calculated to assess sleepiness; mood was evaluated using the Hospital Anxiety and Depression Scale; and quality of life was measured by the Short Form‐36." This suggests 12‐month CPAP usage is intended primary outcome. Results section (text) for CPAP usage reported: "The average compliance was 2.2 hours (51%) longer among participants in the intensive support group than those in the standard support group; this difference was statistically significant between the two groups (Table 13 and Figure 2)." Authors provide no time point reference for this single outcome value, but based on results table, this corresponds to the 3‐month outcome point. The full results table presents CPAP usage outcome data at 1, 3, 6 and 12 month time points. Taken together, this report suggests there was no pre‐determined outcome assessment plan (particularly with regard to time points). |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Chervin 1997.
| Methods | Randomised parallel‐group trial | |
| Participants |
N = 40 participants with OSA (about to start or already receiving CPAP) recruited from clinic. Baseline characteristics: Mean age 51.7. Mean AHI 49.4. ESS 10.9 ± 5.1. Lowest 02 Sat 75.6% (± 14.4). MSLT 6 (± 3.9) Country: USA |
|
| Interventions | No information provided as to the group allocation of all randomised participants. Allocation Ns only available for the 33 participants who completed the study: Intervention group 1 (n = 12), Intervention group 2 (n = 14), control (n = 7). Intervention 1: telephone call each week during trial (max trial time of two months) Intervention 2: two printed documents Control: no additional support Study duration: 8 weeks |
|
| Outcomes |
|
|
| Notes | Two of 33 used Bi‐PAP. Both CPAP‐naive users and those who had been on CPAP before trial were studied. Reading done at enrolment and at between 1 to 2 months after enrolment Difference in AHI between active and control groups at baseline. Trialists included two intervention arms, one educational and one supportive. Intervention 1 (telephone support) vs. Control included in Supportive meta‐analysis. Intervention 2 (educational documents) vs. control included in educational meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not done for treatment group assignment Participants' readout of CPAP machine usage data during telephone call to clinic |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Non‐completers excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | No Information. |
| Other bias | Unclear risk | No Information. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Authors report, "On the basis of a random number table, each subject was assigned to one of three intervention groups."
No reference to allocation concealment method. Key baseline characteristics (age, gender, AHI) were reported for all randomised participants and differences were reported to be non‐significant (no P values presented). BMI comparisons were not reported. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | High risk | No deviations documented; none suspected based upon review. According to the report, 40 participants signed informed consent and were enrolled. Though not explicitly stated by authors, it appears these 40 participants were randomised. However, as noted by authors, "Of the 40 enrolled subjects, five were impossible to reach by phone to establish counter readings and two reported counter readings that were physically impossible, leaving 33 participants (21 men) aged 51.7 +/‐ 11.0 years [ mean +/‐ standard deviation (SD)] who completed the protocol and formed the basis for this report." Therefore, it appears that a per‐protocol analysis was used and no information was presented as to which group(s) the lost participants had been allocated. 17.5% of likely randomised participants were not included in the report. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | Outcome data were not available for 17.5% of likely randomised participants. Neither analyses to correct for bias nor sensitivity analyses were conducted. Yes, reported reasons for exclusion were five were "impossible to reach by phone to establish counter readings" and "reported counter readings that were physically impossible." Authors did not report the group allocation of the seven participants who did not complete the study. Therefore, we do not know if there were differences between intervention groups in proportions of missing outcome data or differences in reasons for missing outcome data. The reported reasons for study non‐completion (and therefore missingness) are suggestive that missingness in the outcome depends on the true value of the outcome. Taken together, it is likely that missingness depended on its true value. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Authors reference that phone reports were compared against objective CPAP usage data, "Discrepancies we found between compliance reported during weekly phone calls and compliance measured by built‐in counters." Each intervention group outcome data ascertained via automated CPAP device monitoring. Authors report that device makes were sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. However, participants reported these counter values by phone, which does introduce the possibility of false reporting. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates one outcome time point (range 1‐2 months) was planned. Results section reports one outcome time point. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Dantas 2015.
| Methods | Randomised parallel‐group trial. | |
| Participants |
N = 41 patients diagnosed with OSAS, meeting the criteria for APAP therapy, were randomly allocated to one of two groups: Intervention Group (IG) brief educational intervention (n = 20) using motivational strategies or control group 1 (CG1, n = 21). ('Control Group 2' (CG2) comprised a convenience sample selected from the sleep lab's initial consultations but were not part of the randomisation procedures.). Inclusion criteria: >18 years old, AHI ≥ 15, diagnosis that indicates ventilator therapy, willingness to participate in the study. Exclusion criteria: COPD, neuromuscular disease, heart disease, neurological disease, and patients taking psychotropic drugs. Baseline characteristics: 23% female. Mean age 56.5 (±10). Mean AHI NR. Mean ESS 9.9. Mean BMI 32.9. Country: Portugal |
|
| Interventions | In the Intervention Group (IG) and control group (CG1), two questions were used to gauge the patient's conviction and confidence: "How important to you is the use of the device in your treatment?" and "How confident are you that you can use the device?" The degree of conviction and confidence permitted to establish the stage of change in each patient, which guided selection of specific strategies to be applied in an individual 10‐minute‐long interview. IG: patient's beliefs, expectations, and feelings were assessed and used as guidance for what motivation strategies were utilised. At the end of the intervention, a new interview was scheduled and written information was delivered about OSAS disease and treatment. CG1: participants received only standardised information about APAP (the device and interface) during the 10‐minute interview, regardless of their confidence and conviction scores. "Control Group 2" (CG2) is a convenience sample submitted to standard procedures, which was not part of the randomisation procedures. CG2 is excluded from review. Study duration: 2 months |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a list of random codes generated by the Excel 2007 program…" |
| Allocation concealment (selection bias) | Unclear risk | Published report does not mention allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study had one "drop‐out" from CG1, therefore outcome data was available for nearly all randomised participants. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, but it is clear that the published reports include all expected outcomes. No evidence that multiple analyses (e.g. different adherence 'thresholds') were conducted. |
| Other bias | Low risk | No baseline imbalances or reported deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Authors report: "Using a list of random codes generated by the Excel 2007 program..."
Published report does not mention allocation concealment. Key baseline characteristics (age, gender, BMI, AHI) were reported and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. Probably mITT: Only 1 dropout reported, in control group 1. Results table Ns are the same as randomised Ns, but unclear if dropout data was included (ITT) in outcome calculation or excluded (mITT). |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Outcome data reported for either all randomised participants (n = 61) or all but one (n = 60). |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. Methods section indicates that multiple time points planned; each planned outcome reported. Methods section indicates that one, commonly‐employed threshold adherence definition was planned; this outcome was reported in Results. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | The primary potential concern is that allocation concealment methods were not described by the authors. Thus, selective enrolment cannot be definitively excluded. |
DeMolles 2004.
| Methods | Randomised parallel‐group study. Methods of randomisation not reported | |
| Participants |
N = 30 patients being started on CPAP for OSAS. Inclusion criteria: starting nasal CPAP therapy; > 18 years; English‐speaking; AHI > 15 Exclusion criteria: prior CPAP use. Baseline characteristics: Mean age 46. Mean BMI 38. Mean AHI 40. Functional Outcomes of Sleep Questionnaire: TLC: 15.3, Control: 13.8 Country: USA |
|
| Interventions | Participants were randomised to telephone‐linked communications technology (TLC, n = 15) versus usual care (UC, n = 15). UC: Described as usual medical care, patient education and demonstration of equipment use. TLC: UC plus computerised digitised human speech programme. TLC asks questions designed to elicit information from participant regarding adherence, education and reinforcement. Study duration: 8 weeks |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants aware of treatment group assignment Intervention involved communication regarding participant's CPAP machine usage |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All completed |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: "At the conclusion of a baseline examination (see the description of study measures subsequently), eligible participants were randomised to either TLC and usual medical care or usual medical care alone....A total of 30 participants were enrolled (15 in each group). All participants completed a 2‐month follow‐up evaluation." No reference to random component or allocation concealment method. Key baseline characteristics (age, BMI, AHI) were reported for all randomised participants and differences were non significant (P values reported), consistent with chance. Gender was not reported, but study appears to have taken place at a VA medical centre in early 2000s, which suggests strong possibility that all participants were male. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No reference to blinding. No deviations documented; none suspected based upon review. Authors report that all participants completed 2‐month follow‐up evaluation and did not report any dropouts, withdrawals, or loss‐to‐follow‐up. Ns not provided for outcome tables, but available information suggests ITT or mITT analyses were used. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Authors report, "All participants completed a 2‐month follow‐up evaluation." |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Unclear risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring. Unable to confirm with study author that distribution of CPAP device makes did not differ between intervention arms. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | High risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates one outcome time point was planned. Results section reports one outcome time point. However, the authors suggest (in results) that the trial may have been longer in the following statement: "The 15 patients in the TLC‐CPAP group participated in the trial for a mean of 9.2 weeks." No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Diaferia 2017.
| Methods | Randomised parallel‐group study. | |
| Participants | For this review, only theN = 49 (male) participants with OSAS Inclusion criteria: men aged 25‐65 years, BMI < 35 kg/m2, confirmed OSAS diagnosis (via polysomnographic criteria). Exclusion criteria: female gender (excluded "since hormonal decline in the menopausal phase could lead to loss of muscle mass, causing a bias in the study"), other sleep disorders, previous treatment for OSAS, serious or decompensated clinical or psychiatric medical illnesses, such as CHF, cardiomyopathy, chronic obstructive pulmonary disease, chronic active hepatitis, liver cirrhosis with severe symptoms, myasthenia gravis, demyelinating disease, motor neuron disease, depression, schizophrenia, obsessive compulsive disorder, disorder anxiety, bipolar disorder, eating disorder, attention deficit disorder, and hyperactivity; patients who used alcohol, stimulants or sedatives; and patients with grade III or IV palatine tonsils, grade II or III septal deviation, or evident micrognathia. Baseline characteristics: 0% female. Mean age 46.9 (±9.9). Mean AHI NR. Mean ESS 12. Mean BMI 28.3. Country: Brazil |
|
| Interventions | Participants were randomised to 2 of 4 study groups were considered: CPAP only (n = 27) or CPAP + myofunctional therapy (MT, n = 22). Full study had 2 additional arms: placebo myofunctional therapy (n = 24) and myofunctional therapy (n = 27) in addition to those noted above for this review. *CPAP only: standard care, including attending a PSG to determine optimal pressure of CPAP *CPAP + MT: combination of orofacial muscle training and standard CPAP treatment. [Placebo: consisted of exercises without therapeutic function (relaxation and stretching of the neck muscles). MT alone: includes soft palate, tongue, and facial muscle exercises and stomatognathic function exercises. Aimed at toning the oropharynx muscles groups, optimising muscle tension mobility, and adjusting the position of the soft tissues and movements including chewing, sucking swallowing and breathing.] Study duration: patients underwent evaluations before and after 3 months of treatment, and after 3 weeks wash‐out period. |
|
| Outcomes |
|
|
| Notes | * Only CPAP only and CPAP + Myofunctional therapy groups included in Review/meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prior to treatment, the patients were divided randomly into four groups." Trialists provided no information regarding random component used in sequence generation or on how sequence concealment was achieved. |
| Allocation concealment (selection bias) | Unclear risk | Trialists provided no information regarding how sequence concealment was achieved. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The numbers of participants randomised to each study arm are not reported |
| Selective reporting (reporting bias) | Low risk | NCT brief summary indicates that assessments at baseline, after treatment and after 21‐day washout would include 'use of CPAP.' Published report (Diaferia 2017) does report 1 week, 1 month and 3 month CPAP usage. |
| Other bias | Low risk | No baseline imbalances or reported deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Authors report: "Prior to treatment, the patients were divided randomly into four groups." Trialists provided no information regarding random component used in sequence generation or on how sequence concealment was achieved. No baseline differences in age, age, BMI, ESS. AHI not included in baseline characteristics table or in textual description of baseline comparison across groups. The results table (5) does report AHI before and after treatment for each group. Baseline differences are present, but statistical significance was not tested. For the intervention arms of interest for our review (CPAP only, CPAP + Myofunctional therapy), those baseline differences are likely consistent with chance. Baseline differences are larger in the other two arms (control, myofunctional therapy only), but likely still consistent with chance. Minimal information provided regarding randomisation procedures. No explicit documentation regarding random component or allocation concealment. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | High risk | NCT01289405 trial information indicates double masking (participant, investigator). Published report indicates only, "During the clinical assessment, the evaluators were blinded." Thus, no evidence that participants or research staff were blind to intervention assignment. Morever, blinding would be difficult given the nature of the interventions. No deviations documented; none suspected based upon review. No information as to whether participants were analysed in the groups to which they were originally assigned since no information was provided as to the original randomisation Ns. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | The numbers of participants randomised to each study arm are not reported. In Results, authors write: "The flowchart of patient selection during this study started with 140 patients with 40 patients failing to complete the study. The 100 patients who finished the study protocol had been distributed to placebo group (N = 24), myofunctional therapy group (N = 27),CPAP group (N = 27), and combined group (N = 22)." There is no flowchart.
Assuming 140 participants were randomised (most likely/rational scenario), a substantial proportion (28.6%) are missing outcome data. Authors provided no information regarding reasons for missing outcome data. Therefore, missingness could depend on true outcome value. The numbers of participants randomised to each study arm are not reported. Therefore, the proportions of participants with missing outcome data in each group cannot be calculated or compared to determine if differences in proportions are significant. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Low risk |
NCT01289405 description did not list CPAP adherence under planned primary or secondary outcomes at 90 day endpoint. NCT brief summary indicates that assessments at baseline, after treatment and after 21‐day washout would include 'use of CPAP.' Published report (Diaferia 2017) does report 1 week, 1 month and 3 month CPAP usage. All planned time points reported. Multiple analyses (e.g. variable adherence 'thresholds') not conducted. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Falcone 2014.
| Methods | Randomised, parallel‐group study. | |
| Participants |
N = 206 newly diagnosed patients with OSA Inclusion criteria: newly diagnosed OSA, AHI ≥15 events/hour, with or without daytime symptoms. Exclusion criteria: COPD, any global respiratory failure, central sleep apnoea syndrome, previous diagnosis of congestive heart failure or cardiomyopathy, any chronic neurological disorder, any severe mental or psychological impairment. Baseline characteristics: 25% female. Mean age 61.3. Mean AHI 54. Mean ESS 11.2. Mean BMI 32.1. Country: Italy |
|
| Interventions | Participants were randomised into educational support (ES, n = 103) or standard support group (SS, n = 103). SS: sleep medicine physician provided each participant with a full explanation (˜10 minutes) of the need for and benefits of CPAP. Prior to CPAP titration the participants received education regarding CPAP operation, mask placement, and a 20‐minute period of auto‐CPAP exposure. ES: in addition to standard support, each educational support group subject viewed 2 consecutive PSGs on the computer screen: the first recorded during a standard diagnostic overnight polysomnography, and the second during a full‐night polysomnography with nasal CPAP. The participant's attention was drawn only to the flow and oxyhaemoglobin saturation curves. Study duration: 12 months |
|
| Outcomes |
|
|
| Notes | * Indicates primary outcome analysed in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was via predetermined balanced blocks, generated by tossing a coin. |
| Allocation concealment (selection bias) | Unclear risk | No reference to allocation concealment method and block size not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants were blinded to the group to which they were allocated, however no details provided as to means by which blinding was carried out/maintained. Moreover, subjective outcome assessors were likely aware of group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 71 of 103 (69%) and 90 of 103 (87%) participants had outcome data at 3‐month endpoint. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, but it is clear that the published reports include all expected outcomes. |
| Other bias | Low risk | No baseline imbalances or reported deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Authors report: "Randomization was via predetermined balanced blocks, generated by tossing a coin."
No reference to allocation concealment method and block size not reported. Key baseline characteristics (age, gender, BMI, AHI) were reported for all randomised participants and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Authors report: "The participants were blinded to the group to which they were allocated." No details provided as to means by which blinding was carried out/maintained.
Authors report study is single‐blind. No deviations documented; none suspected based upon review. Authors report they performed ITT analysis. However, they also report, "participants who did not return for a follow‐up visit were considered nonadherent and dropped‐out the study, so the Epworth Sleepiness Scale scores and CPAP use data in the Results section are only for the adherent CPAP users." Thus, this appears to represent a mITT analysis. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | 71 of 103 (69%) and 90 of 103 (87%) participants had outcome data at 3‐month endpoint. Neither analyses to correct for bias nor sensitivity analyses were conducted. Authors did not report reasons for missingness (dropouts), thus missingness could depend on true values. There are non‐significant differences in the proportion of missing outcome data between intervention groups. There are no reasons reported for missing outcome data other than "did not return for a follow‐up visit" so uncertain as to whether these reasons differ across groups. Per Cochrane Handbook, 8.13.2.2, "Even if incomplete outcome data are balanced in numbers across groups, bias can be introduced if the reasons for missing outcomes differ." Without specific reasons for missing data, the potential for bias cannot be adequately assessed. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Unclear risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring. Unable to confirm with study author that distribution of CPAP device makes did not differ between intervention arms. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned; each planned outcome reported. Methods did not describe intention to assess threshold‐based adherence outcomes; these were reported for each primary endpoint. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Fox 2012.
| Methods | Randomised parallel‐group study | |
| Participants |
N = 75 adults with moderate‐severe OSA by PSG. Inclusion criteria: adult (≥ 19 years), moderate to severe OSA (AHI ≥ 15) Exclusion criteria: active cardiopulmonary or psychiatric disease, previously treated for OSA, no access to telephone line in bedroom, not able to return for follow‐up Baseline characteristics: 20.1% female. Mean age 53.5 (±11.2). Mean AHI 41.6. ESS 9.8.BMI 32.4. Country: Canada |
|
| Interventions | Participants were randomised to telemedicine intervention (TM, n = 39) or standard care (SC, n = 36). TM: physiological data (PAP adherence, applied PAP, mask leak, residual respiratory events) were downloaded using modem attached to the PAP device and sent across the telephone line each morning. Downloaded information was reviewed every weekday except holidays by the research co‐ordinator, who contacted the participant if poor compliance or other problems with treatment (e.g. mask leak) were detected. Participants were advised over the phone or visited the PAP co‐ordinator. Standard care identical to control group SC: 20‐minute orientation to PAP session and mask fitting. Participants contacted after two days to check adherence and to troubleshoot problems, followed up at four to six weeks and at three months; each time, physiological data downloaded from machines and any problems with treatment addressed. In addition, data downloaded at eight weeks Study duration: 12 weeks |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...sequential numbered envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Envelopes were prepared by one of the study investigators |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding undertaken Intervention involved communication regarding participant's CPAP machine usage |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 'intention to treat approach', high discontinuation rate (control group: 10/36, telemedicine group: 11/39) |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: "Patients were randomised to either standard care or telemedicine (1:1 ratio) using sequential numbered envelopes prepared by one of the authors (JW)."
No reference to random component. Allocation concealment method description inadequate for definitive determination. Key baseline characteristics (age, BMI, AHI) were reported for all randomised participants and differences were reported as non‐significant (P values not presented). Statistical comparison of gender proportions (77.8% vs. 82.0% male in control vs. intervention arms) was not reported. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Unblinded. No deviations documented; none suspected based upon review. Probable mITT. Authors report 'using an intention‐to‐treat approach,' but it is not clear how participants who 'Discontinued CPAP' (Figure 1) were handled (i.e. whether excluded from outcome calculations, as in mITT, or counted as non‐adherent and counted as having 0 minutes/night.) |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Although the authors do not state so explicitly, it seems likely that outcome data were available for all participants, As noted in Methods, "Identical to the standard pathway, all patients were oriented to CPAP, fitted with a mask, and given an auto titrating machine. A modem was attached to the PAP device EncoreAnywhere®, Philips Respironics Inc.)..." Thus, those participants who 'Discontinued CPAP' as per Flow Figure 1, probably did not have missing data but had 0 minutes/night. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Low risk | ClinicalTrials.gov entry (NCT00561860) indicates that the original primary outcome measures were the same as the current primary outcome measures, "CPAP compliance (3 months) and overall cost of patient care [ Time Frame: 3 months ]" (submitted 20nov2007). Final data collection date for primary outcome was Sep 2010. Publication year 2012. NCT outcome is the same as that presented in published report. Likely that analysis plan finalised before unblinded outcome data available for analysis. Methods section indicates one outcome time point (for primary adherence outcome) was planned. Results section reports one outcome time point. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Hoet 2017.
| Methods | Randomised, parallel‐group study. | |
| Participants |
N = 46 patients with a recent diagnosis of moderate to severe OSAS Inclusion criteria: at least 18 years old, recently diagnosed with OSAS (AHI ≥20/hours). Exclusion criteria: previous exposure to CPAP therapy, mixed/predominantly central sleep apnoea, language barriers, cognitive or psychiatric disorders making it difficult to comprehend information regarding CPAP therapy and provide informed consent, significant comorbidities such as severe COPD or hypoventilation syndromes. Baseline characteristics: 63% female. Mean age 56.6 (±13.5). Mean AHI 49.5. Mean ESS 11. Mean BMI 31.5. Country: Belgium |
|
| Interventions | Participants were randomised to usual care (UC, n = 23) or telemonitoring (TM, n = 23) group. TM: in addition to usual care, telemonitoring device was attached to CPAP machines. Via this device, sleep laboratory technical staff analysed participant data and contacted patients in the case of air leaks, residual AHI >10/hours, or CPAP use less than 3 hours in three consecutive days UC: group educational session 1 month after CPAP initiation, and a visit to the pneumologist scheduled and 1.5 and 3 months after CPAP initiation. Study duration: 3 months |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors report: "..patients were randomised in permuted blocks between usual care or TM for CPAP follow‐up." Trialists provided no information regarding random component used in sequence generation or on how sequence concealment was achieved. |
| Allocation concealment (selection bias) | Unclear risk | Trialists provided no information regarding random component used in sequence generation or on how sequence concealment was achieved. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No evidence of blinding of participants, personnel or outcome assessors. Study only had objective outcomes, so lack of blinding is not likely affect outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data available for 37 of 46 (80.4%). |
| Selective reporting (reporting bias) | Low risk | NCT02773953 entry: Secondary outcome measure, daily use of CPAP at 3 months, submitted to clinicaltrials.gov on 13 May 2016. Probably specified prior to unblinded outcome data available for analysis. |
| Other bias | High risk | Intervention group composition was 83% female while control group was 43% female, P = 0.0076. Gender is a potentially key prognostic factor in compliance and the between‐group difference is likely large enough to result in bias in the intervention effect estimate. No deviations from intended intervention reported or suspected. |
| Bias arising from the randomisation process (ROB2, primary outcome) | High risk | Authors report: "..patients were randomised in permuted blocks between usual care or TM for CPAP follow‐up." Trialists provided no information regarding random component used in sequence generation or on how sequence concealment was achieved. Intervention group composition was 83% female while control group was 43% female, P = 0.0076. Gender is a potentially key prognostic factor in compliance and the between‐group difference is likely large enough to result in bias in the intervention effect estimate. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. mITT used. Authors report, "During the 3‐month study period, four patients were lost to follow‐up in the TM group and three patients in the UC group. Two patients in the TM group had dysfunction of the TM system. Final analyses were performed on data for the remaining 37 patients (Fig. 1)." |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | Outcome data available for 37 of 46 (80.4%). Neither analyses to correct for bias nor sensitivity analyses were conducted. Loss to follow‐up could be related to participant health status. There are differences in proportion missing between outcome groups (intervention group > control group). There are no reasons provided for the loss‐to‐follow‐up in intervention (n = 3) or control (n = 4) groups. Equipment failure (n = 2) occurred only the intervention group. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Unclear risk | No information |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Low risk | NCT02773953 entry: Secondary outcome measure, daily use of CPAP at 3 months, submitted to clinicaltrials.gov on 13 May 2016. Probably specified prior to unblinded outcome data available for analysis. Per NCT entry, a single time point planned/analysed. Per NCT entry, multiple analyses (e.g. variable adherence 'thresholds') not conducted. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Hoy 1999.
| Methods | Randomised, parallel study. Method of randomisation not reported. ITT | |
| Participants |
N = 80 patients with SAHS. Inclusion criteria: AHI ≥ 15, plus daytime sleepiness or two other major symptoms of the syndrome; resident within 50 miles of Edinburgh Exclusion criteria: prior use of CPAP; coexisting COPD, asthma or neurological problems Baseline characteristics: 2.5% female. Mean age 51 (±11). Mean AHI 58. Mean ESS 13. Mean BMI 33. Country: UK (Scotland) |
|
| Interventions | Participants were randomised into usual care (UC, n = 40) or Telemonitoring (TM, n = 40). TM: full explanation of need for and benefits of CPAP by sleep physician, 20‐minute video education programme, given mask to try for 20 minutes, titration of CPAP pressure overnight with following day discharge, nurses telephoned on days two and 21, reviewed in hospital at one, three and six months. Initial education at home with partner, two extra nights in hospital, sleep nurses' home visits to participant and partner at seven, 14 and 28 days and four months after starting CPAP UC: full explanation of need for and benefits of CPAP by sleep physician, 20‐minute video education programme, given mask to try for 20 minutes, titration of CPAP pressure overnight with following day discharge, nurses telephoned on days two and 21, reviewed in hospital at one, three and six months Study duration: 6 months |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each participant was randomly assigned with predetermined balanced blocks generated by tossing a coin |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single‐blind: "Patients were blinded to the group to which they were allocated" Not enough information available to ascertain awareness of CPAP machine usage |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Data were analysed on an intention‐to‐treat basis" |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Authors report, "Randomization of each patient was done with predetermined balanced blocks generated by tossing a coin. Patients were blinded to the group to which they were allocated." No reference to allocation concealment method. Key baseline characteristics (age, BMI, AHI) were reported for all randomised participants and differences were insignificant (P values reported), consistent with chance. Randomised participants included only 2 females (78 males); authors did not report treatment allocation by gender. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. ITT |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Authors report, "Seven of the 80 patients (four receiving standard and three intensive support) were unavailable for daytime function retesting at 6 mo; the four patients in the standard support group had stopped using CPAP, one patient in the intensive support group died of lung carcinoma diagnosed after randomisation, another stopped using CPAP, and one defaulted from daytime testing at 6 months. All 80 patients had their CPAP usage over the 6‐mo trial period recorded and analysed." |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned; each planned outcome displayed graphically but only final (6‐month) outcome reported in table. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Hui 2000.
| Methods | Randomised, parallel‐group study | |
| Participants |
N = 108 patients with newly‐diagnosed OSA. Inclusion criteria: diagnosis of OSA (AHI > 10 and subjective daytime sleepiness) Exclusion criteria: none reported. Baseline characteristics: 10% female. Mean age 45 (±11). Mean AHI 48. Mean ESS 12.8. Mean BMI 30. Country: China (Hong Kong) |
|
| Interventions | Participants were randomised to basic CPAP support (BS, n = 54) or augmented support (AS, n = 54) AS: 10‐minute CPAP education programme by respiratory nurse, brochure on OSA and CPAP treatment in Chinese, short trial CPAP therapy with comfortable mask for 30 minutes, CPAP titration on second night of study by AutoSet, nursing support following day, follow‐up by nursing staff and physician at 1 and 3 months. Locally produced 15‐minute videotape, additional nurse led 15‐minute educational session, review by physicians at weeks one and two, respiratory nurse telephone call on days one and two, weeks one, two, four, eight and 12 BS: 10‐minute CPAP education programme by respiratory nurse, brochure on OSA and CPAP treatment in Chinese, short trial CPAP therapy with comfortable mask for 30 minutes, CPAP titration on second night of study by AutoSet, nursing support following day, follow‐up by nursing staff and physician at 1 and 3 months. Study duration: 12 weeks |
|
| Outcomes |
|
|
| Notes | 91 participants had to purchase or rent their machines. 17 participants (10 in AS group and seven in BS group) qualified for state support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not specified. Participants provided subjective CPAP machine usage data |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were analysed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | High risk | Only information provided as to the randomisation procedures used was within Methods: "The participants were randomised into two arms, with group 1 receiving basic CPAP education and support and group 2 receiving augmented education and support."
No reference to random component or allocation concealment method. Some key baseline characteristics (BMI, AHI) were reported for all randomised participants and differences were insignificant (P values reported), consistent with chance. However, proportions of age and gender were not reported by intervention arm nor were statistical comparisons reported. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No blinding. No deviations related to experimental context documented; none suspected based upon review. ITT. Authors report, "Data were analysed on an intention‐to‐treat basis." However, they also report, "All the patients returned for follow‐up, but there was a technical problem with the Aria/Encore software, resulting in missing CPAP compliance data for 11 of the 108 patients (2 in the BS group and 9 in the AS group) at 3 months." They do not report how missing outcome data were handled. Results table 2 indicates that outcomes are reported on all randomised Ns, so appears to be ITT. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Authors report, "All the patients returned for follow‐up, but there was a technical problem with the Aria/Encore software, resulting in missing CPAP compliance data for 11 of the 108 patients (2 in the BS group and 9 in the AS group) at 3 months." Thus, outcome data were available for 11 of 108 (89.8%) of randomised participants. Neither analyses to correct for bias nor sensitivity analyses were conducted. Missing outcome data occurred for documented reasons ('technical problems with Aria/Encore software') that are unrelated to the outcome. These failures were differentially distributed across intervention arm. In Discussion (limitations), authors report, "There was also a technical failure with the Aria/Encore software, resulting in missing CPAP compliance data for two patients in the BS group and nine patients in the AS group at 12 weeks." |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned; each planned outcome reported. Methods section indicates that multiple analyses of CPAP adherence outcomes were planned and all were reported in Results. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Hwang 2017.
| Methods | Cluster‐randomised parallel‐group study | |
| Participants |
N = 1455 patients with suspected OSA were randomised to four study arms, by class‐based (cluster) randomised design. This study used the existing home‐based testing triage structure at the trialists institution. As they report, "Most patients are referred by primary care physicians, and a sleep medicine physician triages appropriate patients to home sleep apnoea testing after review of the referral information and electronic health record chart. HSAT classes (up to 13 people) are led by a sleep‐trained respiratory therapist and sleep technologist and provide interactive OSA education and individualised HSAT setup. After a one‐night test, each patient returns for an individual appointment with a respiratory therapist to review the results. Those with OSA are recommended to undergo a 1‐week CPAP trial followed by an individual return appointment with a respiratory therapist to review CPAP data and patient experience. Patients willing to commit to CPAP therapy are immediately dispensed a device; otherwise CPAP troubleshooting or alternative treatments are discussed." This trial enrolled Consecutive patients referred to the Kaiser Permanente Fontana Sleep Disorders Center (Fontana, CA) for evaluation of suspected OSA and triaged to HSAT between November 2014 and August 2015.To conform to the sleep centre's usual care procedures, groups of patients were randomised, with all participants in each HSAT class following the same treatment arm. Inclusion criteria: at least 18 years of age, no previous sleep testing or trial of OSA therapy, eligible for HSAT. Exclusion criteria: at risk of other sleep disorders (e.g. severe insomnia), significant cardiopulmonary disease (e.g. heart failure, chronic respiratory failure), or English not preferred language. Baseline characteristics: 51% female. Mean age 49.1 (±12.5). Mean AHI 22.7. Mean ESS 9.1. Mean BMI 34. Country: USA |
|
| Interventions | Classes (and all participants in each class) were randomised (1:1:1:1) to one of four arms: 1) web‐based OSA education (Tel‐Ed, n=380), 2) telemonitoring and automated feedback (Tel‐TM, n = 375), 3) Tel‐Ed + Tel‐TM (Tel‐Both, n = 346), and 4) usual care (UC, n = 354) using a four‐arm, randomised, factorial design. Usual care: all patients attended a 1‐hour, small‐group education class with HSAT set‐up. After the trial, those willing to continue CPAP were prescribed therapy and scheduled for a 3‐month follow‐up appointment. Tel‐Ed: education about the pathophysiology of OSA, health‐related risks, impact on daytime vigilance, introduction to CPAP therapy. For patients eventually determined to have OSA, a link to a second education program was emailed. This focused on how to use CPAP, potential benefits, methods of acclimating, and equipment care instructions. Education sessions were interactive and self‐paced. Tel‐TM: Intervention based on automatic processing of device data. During the 3‐month study period, if CPAP usage thresholds were met, a message was automatically sent to the patient providing encouragement to improve use or positively reinforcing successful adherence. Tel‐both: patients received Tel‐Ed and Tel‐TM Study duration: 90 days |
|
| Outcomes |
|
|
| Notes | Trialists included three intervention arms. One arm was educational (Tel‐Ed), one was Supportive (Tel‐TM) and the third was Mixed (Tel‐Both). These were compared to control in respective meta‐analyses (i.e. Educational, Supportive, Mixed). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Author's 'group randomisation' does not conform to individual or block randomisation schemes. |
| Allocation concealment (selection bias) | Unclear risk | No reference to allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors report, "Overall, 66% showed up for the HSAT class and were tested, and 81.1% of those tested were diagnosed with OSA (AHI>5). Five hundred and fifty‐six patients (38.2% of all randomised patients; 71.7% of all patients with OSA) were eventually prescribed CPAP, all of whom had 90‐day usage data to be included for analysis." |
| Selective reporting (reporting bias) | Low risk | NCT02279901: Original/current primary outcome measures (unchanged from original submission on 30 Oct 2014) was: 3‐month average CPAP use/night (experimental pathway vs. traditional pathway). Secondary measures (30 Oct 2014, unchanged from original submission): Difference in 3‐month average CPAP use/night (experimental vs. experimental), ESS, FOSQ‐10, adherence to provider encounters (no‐show), healthcare utilisation. |
| Other bias | Unclear risk | Baseline characteristics for all randomised participants presented in tabular form by authors but no statistical comparison reported. No deviations from intended intervention reported or suspected. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: "To conform to the sleep centre's usual care procedures, groups of patients were randomised, with all participants in each HSAT class following the same treatment arm. Classes were randomised (1:1:1:1)...both). Randomization was performed for each HSAT class (not in blocks), using a computerized random number generator."
In their discussion, authors further note, "...group rather than individual randomisation was performed; nevertheless, treatment arm baseline characteristics were similar and within‐group correlations were taken into account in analyses."
Author's 'group randomisation' does not conform to individual or block randomisation schemes. No reference to allocation concealment method. Baseline characteristics for all randomised participants presented in tabular form by authors but no statistical comparison reported. Authors do report that "baseline characteristics for those prescribed CPAP were similar between the four arms." |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Authors report, "Clinicians providing routine care and study analysts were blinded to study arm assignment." No deviations documented; none suspected based upon review. Probable mITT. There is one discrepancy in Tel‐Ed group n = 164 in Study flowchart and baseline characteristics table 2 and the outcome data table n = 163. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Unclear risk | Authors report, "use), 162 HSAT classes—accounting for 1,455 patients—were randomised (Figure 1). Overall, 66% showed up for the HSAT class and were tested, and 81.1% of those tested were diagnosed with OSA (AHI>5). Five hundred and fifty‐six patients (38.2% of all randomised patients; 71.7% of all patients with OSA) were eventually prescribed CPAP, all of whom had 90‐day usage data to be included for analysis." Neither analyses to correct for bias nor sensitivity analyses were conducted. Missing outcome data occurred for documented reasons (declined CPAP) that could be related to the outcome. Across intervention arms, the proportion of participants diagnosed with OSA (in evaluation period), who accepted CPAP therapy during the evaluation phase and, therefore, for whom 90 day CPAP usage data were available/analysed ranged from 68.3% (Tel‐TM) to 76.3% (Tel‐Ed) groups. UC and Tel‐Both proportions were 71%. Thus, as noted by study authors, 'drop‐out' rates were similar across groups. Additionally, there is no evidence that reasons for dropout differed across intervention arms and cited reasons for all dropouts in author's study flowchart is 'declined CPAP' and is noted in text to be 'elected for non‐CPAP therapies'. Thus, due to similar proportions and cited reasons across intervention arms, missing outcome data are unlikely to lead to bias in the estimated effects of the intervention. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Low risk | NCT02279901: Original/current primary outcome measures (unchanged from original submission on 30 Oct 2014) was: 3‐month average CPAP use/night (experimental pathway vs. traditional pathway). Secondary measures (30 Oct 2014, unchanged from original submission): Difference in 3‐month average CPAP use/night (experimental vs. experimental), ESS, FOSQ‐10, adherence to provider encounters (no‐show), healthcare utilisation. Primary outcome identical in protocol and in published report. Some secondary outcomes not noted in protocol but are in published report. One outcome time point (for primary adherence outcome) planned, one outcome time point reported. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Lai 2014.
| Methods | Randomised, parallel‐group study | |
| Participants |
N = 100 patients with newly diagnosed OSA. Inclusion criteria: at least 18 years old, newly diagnosed OSA, AHI ≥ 5, receiving in‐laboratory auto‐CPAP titration for the first time, no prior OSA or CPAP education classes. Exclusion criteria: CSA, periodic leg movement disorders, COPD, pregnancy, psychiatric illness on treatment, cognitive impairment, illiteracy, unstable health conditions, unable to attend the education session before discharge from Sleep Disorders Centre, scheduled for OSA follow‐up in other hospitals, or participating in another clinical trial. Baseline characteristics: 17% female. Mean age 51.98 (±10). Mean AHI=29.42. Mean ESS=9.25. Mean BMI=28.96. Country: China (Hong Kong) |
|
| Interventions | Participants were randomised to usual care (UC, n = 51) or UC + brief motivational enhancement program (ME, n = 49). UC: 15‐minute talk to teach basic operation of the CPAP device and titration procedure. Medical officer explanation of OSA, participant results, and prescribed treatment. Nurse advice on importance of CPAP therapy and care of accessories. ME: in addition to receiving UC, ME arm received an intervention designed to enhance the participant's perception of the risk of OSA, confidence in the ability to apply CPAP treatment (self‐efficacy), and association of their behavior to the desired outcome (adherence) or outcome expectancy, including: a session on morning after CPAP titration, telephone call on day 2 of CPAP use, providing early follow‐up, 25‐minute video about CPAP education (including real‐life experience of a current CPAP user), 20‐minute patient centred face‐to‐face motivational interview, a 10‐minute follow‐up phone call on day 2 of CPAP. Checklists for interview and phone follow‐up were used to ensure treatment fidelity. Duration: 3 months. |
|
| Outcomes |
All outcomes assessed at 1 and 3 months. |
|
| Notes | * Indicates primary outcome analysed in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trialists give extensive explanation for randomisation procedures, including the use of a computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | "The allocation concealment was achieved with sequentially numbered opaque sealed envelopes." |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants to group allocation (but were not aware that adherence would be measured), personnel or outcome assessors. Although this is unlikely to affect adherence, study also investigated subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One withdrawal from each group, results in less than 10% missing outcome data. |
| Selective reporting (reporting bias) | High risk | Publication (2014), earlier abstract (2013) and ClinicalTrials.gov entry available and reviewed. ClinicalTrials.gov entry (NCT01173406) indicates the originally‐specified primary outcome measure was 4‐week CPAP usage (submitted 30 Jul 2010). This was amended (18 Oct 2018) to 12‐week CPAP usage (the original secondary outcome, submitted 30 Jul 2010). Updated (18 Oct 2013) secondary/other outcomes were 12‐week daytime sleepiness, 12‐week self‐efficacy measure for sleep apnoea. This information suggests the possibility that reported numerical results were selected on the basis of results. |
| Other bias | Low risk | No baseline imbalances or reported deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Low risk | Authors report, "Randomization sequence was created using a computer‐generated randomisation program (www.randomization.com). The randomisation was stratified into three severity groups: AHI ≥5 to < 15, AHI ≥ 15 to < 30, and AHI > 30, and with 1:1 allocation using a block size of 6." Authors provide additional information in the supplementary Appendix: "The application of stratified randomisation was used to prevent any significant difference in OSA severity of the recruited participants between two groups. The allocation concealment was achieved with sequentially numbered opaque sealed envelopes. Corresponding envelopes were opened only after the enrolled participants completed all baseline assessments. The randomisation and allocation concealment procedures were performed by a research staff who was not involved in the recruitment process, data collection or education intervention."
The use of a fixed, relatively small (n = 6) block sizes would have made it possible for staff involved in allocation procedures to predict the last intervention assignment within each block. However, trialists report that randomisation and allocation concealment procedures were performed by a research staff member who was not involved in recruitment, data collection or intervention. Key baseline characteristics (age, gender, BMI, AHI) were reported and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Supplementary Appendix: "participants were asked to participate in an intervention that could improve their ability to use CPAP but they were not informed that this was an adherence study or the group to be assigned to. Data collectors were kept blinded to the allocation procedures and were not involved in the education intervention." No deviations documented; none suspected based upon review. ITT. In supplementary appendix, the authors report, "The intention–to‐treat (ITT) principle was adopted to examine the efficacy of brief motivational enhancement education program. Specifically, patients were analysed as randomised and missing values were replaced by the last observed value. For the two dropouts, carry‐forward data were computed to replace the missing values and the CPAP usages was counted as zero hours." |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Information not available |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | High risk | Publication (2014), earlier abstract (2013) and ClinicalTrials.gov entry available and reviewed. ClinicalTrials.gov entry (NCT01173406) indicates the originally‐specified primary outcome measure was 4‐week CPAP usage (submitted 30 Jul 2010). This was amended (18 Oct 2018) to 12‐week CPAP usage (the original secondary outcome, submitted 30 Jul 2010). Updated (18 Oc t2013) secondary/other outcomes were 12‐week daytime sleepiness, 12‐week self‐efficacy measure for sleep apnoea. Final data collection date for primary outcome was April 2012. Published report indicates that outcomes would be assessed at 1 and 3 months. An earlier abstract, long‐term efficacy of motivational interviewing on improving continuous positive airway pressure adherence in obstructive sleep apnoea: A randomised controlled trial [Abstract] presented at the European Respiratory Society Annual Congress, 2013 Sept 7‐11, Barcelona, Spain ( same sample N, same age/ESS means) stated alternate objectives: "This study aimed to examine the long‐term efficacy of a theory‐based behavioral education (BMI‐E) programme on improving CPAP adherence... Primary outcome was to assess CPAP adherence 1 year after receiving BMI‐E programme." Taken together, it is possible that some aspects of the analysis plan were not finalised before unblinded outcome data were available for analysis and there are discrepancies across different reports in the designation of primary and secondary outcome time points. The authors' final publication (Lai 2014) does report the original and amended outcome time points (1 and 3 months) report both time point outcomes listed in NCT entry. However, the authors do not report 1‐year average CPAP usage/day outcome (listed as planned primary outcome measure in 2013 abstract) in the published 2014 report. Additionally, the abstract reports only a threshold‐based adherence outcome, one not specified in the NCT entry. Finally, the published report describes multiple additional ways that the outcome was analysed (i.e. proportion of CPAP‐adherent uses and CPAP usage index). This information suggests the possibility that reported numerical results were selected on the basis of results. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Lewis 2006.
| Methods | Prospective, single‐blinded interventional study | |
| Participants |
N = 72 patients with newly‐diagnosed SAHS immediately prior to CPAP titration. Inclusion criteria: diagnosis of OSA (based on home sleep study) and subjective daytime sleepiness Exclusion criteria: not reported Baseline characteristics: 13.8% female. Mean age 51.4 (±8.6). Mean AHI 42.5. Mean ESS 15.7. Mean BMI 36.5. Country: UK |
|
| Interventions | Participants were randomised to standard support (SS, n = 36) or intensive support (IS, n = 36) group. IS: 20‐minute educational video about SAHS. Telephone interview by research assistant between days two and five after CPAP issued to identify early problems and advise. Extra appointment to see sleep physician within seven to 14 days after being issued CPAP. Further appointment with sleep physician at 1, 6, and 12 months SS: participants provided telephone number for support within office hours. Sleep physician reviewed participants at 1, 6, and 12 months Study duration: 52 weeks |
|
| Outcomes |
|
|
| Notes | Only 20/36 participants in the intervention group watched the educational video tape. Eight of the 17 defaulters returned machines at different times of the year and had negligible hours of use. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using block tables |
| Allocation concealment (selection bias) | Low risk | 'The sequence of group assignment was indeed concealed from the investigators undergoing the screening and assessments, especially those recording/analysing machine hours' |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single‐blinded: participant unaware of what 'intensive' or standard support comprised 'The CPAP clock‐timers were hidden with a plastic strip. Patients were not informed about the timers, and all covers were intact at each review; both patients and those recording clock‐timers were unaware of group allocation' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Non‐completers not included in analysis of usage data |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: "Consecutive attenders at auto‐titration were randomised, using block tables, to receive either intensive (intervention group) or standard support (control group)." Key baseline characteristics (age, gender, BMI, AHI) were reported for all randomised participants and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Authors report, "Patients were not informed about the timers, and all covers were intact at each review; both patients and those recording clock‐timers were unaware of group allocation." No deviations documented; none suspected based upon review. Appears to be mITT. Authors report, "Data missing because of non‐attendance were excluded from analysis." In Results text, authors reference '17 defaulters,' corresponding to the 17 participants missing from the Results graph, Figure 2 (n = 25 control, n = 30 intervention participants). |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | At 6‐month endpoint, 32 of 36 and 26 of 36 of intervention and control groups, respectively, had CPAP usage data, corresponding to 80.6% of participants. At 12 months, 74.3% of outcome data were available. Neither analyses to correct for bias nor sensitivity analyses were conducted. Authors note, "Non‐attenders were noted, but no further data could be reliably calculated. We did not utilise further statistical techniques for longitudinal data with missing information, as it is clear that data is not missing by random." Authors report, "Eight out of the 17 defaulters (or their relatives) returned their machines at various times in the year. All had negligible hours on the clock‐timers, but we could not calculate average nightly use for these patients because we could not tell when they stopped using their machines." Authors did not report further information as to the reasons participants defaulted or returned their machines. Thus, missing outcome data occurred due to loss to follow‐up, which means that missingness could depend on true value. Authors report, "Despite likely selection bias (poor users dropping out in the standard group but remaining in the intervention group), there were still fewer reporting problems, more reporting enthusiasm and lower mean side‐effects scores in the intervention group at one year." There were differences between groups in proportion missing outcomes (control > intervention) at endpoints. No reasons were available for participants who defaulted, so comparison of reasons for missingness could not be conducted. Thus, as authors acknowledge, it is likely that missingness in the outcome depended on true value. Additional information provided by authors in their report: Methods (outcomes): "Numbers of patients who did not attend follow‐up, phone for help or require additional interventions were noted at each time point. Those who did not attend follow‐up were sent a written reminder and re‐booked within two weeks of the original appointment. A second missed attendance triggered a phone call by a research assistant or sleep physician, asking these patients to return their CPAP machine if unable to use it. Those who did return their machines with negligible use were referred for other treatment modalities." Statistical Analyses: "...Data missing because of non‐attendance were excluded from analysis." Discussion: "By recording machine use at several points, we could see trends in usage in that eventual non‐attenders tended to have less frequent use on their previous visits (or when they eventually returned their machines). This is particularly important because subsequent differences in non‐attendance rates mean that previous studies recording machine usage only in those who turn up at clinic are prone to reporting bias. We asked all patients to re‐attend clinic, and all patients knew about the merits of CPAP. Non‐attenders were noted, but no further data could be reliably calculated. We did not utilise further statistical techniques for longitudinal data with missing information, as it is clear that data is not missing by random. We now believe that studies measuring group or individual trends in CPAP use should also record attendance rates to provide a more complete clinical picture." |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned; each planned outcome reported. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Mendelson 2014.
| Methods | Randomised, parallel‐group study. Randomisation was stratified by recruitment centre in blocks of 6 participants. | |
| Participants |
N = 107 patients with OSA and a high cardiovascular risk (cardiovascular score > 5% or secondary prevention). Inclusion criteria: age between 18 and 85 years, diagnosed with OSA on diagnostic sleep study (AHI > 15), BMI < 40 kg/m2, cardiovascular risk score > 5%, or being in secondary prevention with a past history of cardiovascular disease. Exclusion criteria: CSA, cardiovascular score < 5%, cardiac failure, history of hypercapnic chronic respiratory failure, incapacitated patients, pregnancy or taking part in another clinical trial. Baseline characteristics: 16.8% female. Mean age 63 (±9). Mean AHI=39. Mean ESS=7.9. Mean BMI=29.9. Country: France |
|
| Interventions | Participants were randomised to telemedicine (n = 54) or standard care (n = 53). Standard care: evaluated at baseline, fitted with a nasal mask and given an auto titrating machine. Patients were contacted after 2 days to ask about adherence and to troubleshoot. After 4 weeks of treatment, patients met with their sleep specialist and data downloaded from machines. After 4 months of treatment, patients consulted their sleep specialist and were re‐evaluated. Telemedicine: in addition to standard care, TM participants were equipped with a smart phone for uploading BP measurements, CPAP adherence, sleepiness, and quality of life data. They received daily pictograms containing health‐related messages. Study duration: 4 months. |
|
| Outcomes |
All outcomes were measured at 4 months only. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were enrolled and followed up by the research team who assigned them to telemedicine or standard care on the basis of a computer‐generated allocation sequence. Randomization was stratified by the recruiting centre in blocks of 6 participants." |
| Allocation concealment (selection bias) | Low risk | "Treatment allocations were prepared by an individual otherwise unaffiliated with the study." |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Though not noted explicitly, outcome data were likely missing for those participants who were excluded, lost to follow‐up or withdrew (enumerated in Figure 2), suggesting that outcome data were available for 79.2% (control) and 74.1% (intervention) at study endpoint. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but it is clear that the published reports include all expected outcomes in accordance with trial's NCT entry. |
| Other bias | Unclear risk | Key baseline characteristics (age, BMI, AHI) were reported for all randomised participants and differences appear non‐significant (overlapping SEs). However, gender proportions (90.7% and 75.5% males in intervention and control arms, respectively) not statistically compared by authors. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Low risk | Authors report, "Participants were enrolled and followed up by the research team who assigned them to telemedicine or standard care on the basis of a computer‐generated allocation sequence. Randomization was stratified by the recruiting centre in blocks of 6 participants. Treatment allocations were prepared by an individual otherwise unaffiliated with the study."
Although allocations were made by an individual unaffiliated with the study, it is not clear if study investigators were aware of the fixed block size. However, given the number of recruiting centres, ability of study investigators at any single centre to predict allocation based on knowledge of block size is deemed to be low. Key baseline characteristics (age, BMI, AHI) were reported for all randomised participants and differences appear non‐significant (overlapping SEs). Gender proportions (90.7% and 75.5% males in intervention and control arms, respectively) not statistically compared by authors. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. ITT. Authors report, "The data for all outcomes were analysed on an intention‐to‐treat basis....The missing values at baseline were replaced by the median of each group, and at follow‐up, they were substituted by data at baseline (method of means bias)." |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | Though not noted explicitly, outcome data were likely missing for those participants who were excluded, lost to follow‐up or withdrew (enumerated in Figure 2), suggesting that outcome data were available for 79.2% (control) and 74.1% (intervention) at study endpoint. Neither analyses to correct for bias (as described in Cochrane Handbook 6.1.6) nor sensitivity analyses were conducted. Missing outcome data occurred due to loss to follow‐up or study withdrawal, both of which could be related to the outcome. Therefore, it is possible that missingness in the outcome was influenced by its true value. Though there is no substantial difference in the missingness proportions across groups, the reported reasons for missingness suggest that missingness depends on the true value and the reasons for missingness differ across groups. As authors note, "...it is possible that telemedicine was perceived as an additional burden associated with the self‐management of BP and CPAP by patients randomised to this group. In fact, there were more dropouts in the telemedicine group than standard care (n = 8, 14.8% vs n = 1, 1.9%, respectively), which is consistent with another study using this type of intervention." |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Authors contacted to verify CPAP make, and provided the following reply: "Author response: "In the study "CPAP treatment supported by telemedicine does not improve blood pressure in high cardiovascular risk OSA patients: a randomised, controlled trial; Sleep 2014;37 MISC1 ‐ SLP(11):1863‐1870B", the CPAP device used was iSleep by BREAS (http://breas.com/products/isleep/). All patients included in this study used the same device." Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Low risk | ClinicalTrials.gov entry (NCT01226641) indicates that the original secondary outcome measure (that which is relevant to our review) was the same as the current primary outcome measure, "CPAP compliance [ Time Frame: week 16 ] the CPAP compliance is assessed in the two groups at week 16 " (submitted 21 Oct 2010). Final data collection date for primary outcome was Jan 2012. NCT outcome is the same as that presented in published report (2014). Published report indicates that the study was conducted between July 2009 and January 2012. Thus, the dates provided indicate that outcomes were finalised before unblinded outcome data would have been available for analysis (i.e. outcome specified soon after start of recruitment period). NCT entry and published report Methods section indicates that one outcome time point (for adherence outcome) was planned and one outcome endpoint reported. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Meurice 2007.
| Methods | Randomised parallel‐group trial. | |
| Participants |
N = 112 participants with severe OSA and no prior treatment for OSA. Inclusion criteria: PSG‐confirmed OSA (AHI > 30), no prior OSA treatment, treated with constant pressure. Exclusion criteria: none reported. Baseline characteristics: Mean age 58 (±11). Mean AHI=58(±25). Authors reported mean ESS and BMI by intervention arm and reported no significant differences. Gender distribution not reported. Country: France |
|
| Interventions | Participants were randomised to Intervention Group 1 (n = 27), Group 2 (n = 27), , Group 3 (n = 27) or Group 4 (n = 26), defined as follows. Intervention group 1: RP + RH Intervention group 2: RP + SH Intervention group 3: SP + RH Intervention group 4: SP + SH (control) Reinforced education by Homecare Network (RH): home visit by technician at installation and further visits for explanation at one week, one month and two and three months of treatment for repetition of education and problem solving Reinforced education by prescriber (RP): written material on CPAP use; explanation of OSA and CPAP with side effects; emphasis on importance of compliance with CPAP and detailed demonstration Standard education by the homecare network (SH): homecare visit to supply the CPAP machine, fit the mask and explain the technique of using the apparatus. CPAP mechanism and method of using the machine and mask were explained. Participant was encouraged to ask questions and could phone at any time to resolve problems Standard education by the prescriber (SP): standard oral explanation of OSA and CPAP, brief demonstration of machine use plus manufacturer's literature. Participant was encouraged to ask questions and clarify misunderstandings. Study duration: 3 months, per protocol. Follow‐up to 52 weeks (intervention administered at outset of study). Data extracted at three months. Authors report "During the remaining 9 months following the initial study design, there was no specific follow‐up protocol and patients benefited from the standard homecare surveillance recommended in the ANTADIR network, with a review every 3 months" |
|
| Outcomes |
|
|
| Notes | Intervention groups 1, 2 and 3 combined for comparison to Control group (4) in meta‐analysis, as recommended in Cochrane Handbook section 16.5.4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: "Patients were consecutively recruited from seven centres and were randomised into the four educational strategies." No reference to random component or allocation concealment method. |
| Allocation concealment (selection bias) | Unclear risk | No reference to allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were available for all randomised participants at the first endpoint (3 months). |
| Selective reporting (reporting bias) | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. |
| Other bias | Unclear risk | Some key baseline characteristics (age, BMI, AHI) were reported and differences were insignificant (P values reported), consistent with chance. However, gender, another key characteristic, was neither reported nor compared across intervention arms. No deviations from intended interventions reported or suspected. |
| Bias arising from the randomisation process (ROB2, primary outcome) | High risk | Only information provided as to the randomisation procedures used was within Methods: "Patients were consecutively recruited from seven centres and were randomised into the four educational strategies."
No reference to random component or allocation concealment method. Some key baseline characteristics (age, BMI, AHI) were reported and differences were insignificant (P values reported), consistent with chance. However, gender, another key characteristic, was neither reported nor compared across intervention arms. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. ITT |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Authors provide CPAP usage outcome data at 3 endpoints. No endpoint was designated as primary by trialists. Data were available for all randomised participants at the first endpoint (3 months); for 96 of 112 (85.7%) at 6 months and for 91 of 112 (81.3%) at 12 months. For the purposes of our review, the 3‐month endpoint was selected. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Unclear risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring. Unable to confirm with study authors that distribution of CPAP device makes did not differ between intervention arms. Authors report, "The choice of the machine used by the patients corresponded to the wishes of the prescribers." Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned; each planned outcome reported. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Munafo 2016.
| Methods | Randomised, parallel‐group study. | |
| Participants |
N = 122 newly diagnosed patients with OSA. Inclusion criteria: age 18–80 years, CPAP‐naïve, confirmed OSA (AHI 5–70) diagnosis based on polysomnography (PSG) or home sleep test, access to and be able to utilise communication technology (text messaging, e‐mail). Exclusion criteria: prominent central apnoea (>20 %), claustrophobia, current use of mandibular repositioning device, other OSA therapy. Baseline characteristics: 31% female. Mean age 51.2 (±11.2). Mean AHI=30.4. Mean ESS=10.5. Mean BMI=33.2. Country: USA |
|
| Interventions | Participants were randomised to standard of care (SOC, n = 64) alone, or SOC + web‐based automated telehealth messaging program (TH, n = 58). SOC: patients were dispensed a CPAP device on Day 0, then contacted via phone on Days 1, 7, 14, 30, and 90. CPAP usage and efficacy data were tracked via the wireless modem attached to CPAP machine. Modem data were accessed via online platform. Frequent phone calls and return clinic visits were provided, as necessary. TH: CPAP device dispensed on Day 0, along with a pamphlet about U‐Sleep, a web‐based application to monitor adherence and message patients and providers via automated series of text messages/emails were triggered by pre‐set conditions. Study duration: 3 months |
|
| Outcomes |
All outcomes measured at 90 days. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: participants subsection, where authors report: "A simple randomisation scheme was used to allocate patients to CPAP treatment plus SOC or TH." Trialists provided no information regarding random component used in sequence generation or on how sequence concealment was achieved. |
| Allocation concealment (selection bias) | Unclear risk | No reference to allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There are differences in the proportion of missing outcome data between intervention groups. There are no reasons for missing outcome data other than 'loss‐to‐follow‐up,' so uncertain as to whether these reasons differ by group. |
| Selective reporting (reporting bias) | Low risk | No protocol available. Published abstract (2014) listed the same primary outcomes, time points. |
| Other bias | Low risk | No baseline imbalances or reported/suspected deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: participants subsection, where authors report: "A simple randomisation scheme was used to allocate patients to CPAP treatment plus SOC or TH." Trialists provided no information regarding random component used in sequence generation or on how sequence concealment was achieved. Key baseline characteristics (age, gender, BMI, AHI) were reported and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. Only observed deviations from planned intervention is non‐compliance with behavioural intervention which is typical of routine care, so unrelated to the experimental context. ITT conducted only for 'Medicare Adherence' definition ‐ "...use for ≥4 hours/night on 70 % of nights during a 30 consecutive‐day period anytime during the first 90 days of initial usage." (p. 779) Authors report, "Primary endpoint analyses were generated for the intention‐to‐treat (ITT) and completed cases (CC) populations. The ITT population included all randomised patients except two who withdrew consent. Patients with no compliance data, and one patient who never enrolled in the U‐Sleep program, were considered non‐adherent to CPAP; for those lost‐to‐follow‐up, adherence results for the last available assessment were used. The CC population included patients who completed the study according to the protocol. Additional analyses were conducted in the CC population without imputation for missing values." |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | Authors report: "Primary endpoint analyses were generated for the intention‐to‐treat (ITT) and completed cases (CC) populations. The ITT population included all randomised patients except two who withdrew consent. Patients with no compliance data, and one patient who never enrolled in the U‐Sleep program, were considered non‐adherent to CPAP; for those lost‐to‐follow‐up, adherence results for the last available assessment were used. The CC population included patients who completed the study
according to the protocol. Additional analyses were conducted in the CC population without imputation for missing values." (p. 779) Reviewer note: Neither analyses to correct for bias nor sensitivity analyses were conducted. Authors note: "There are some limitations to this study. Firstly, the ability of the study to detect a significant difference in adherence and daily usage between the TH and SOC groups was reduced by the exclusion of 18 patients from the final analysis and the high adherence rate in the SOC group. Of the 18 patients with insufficient data, 12 were from the TH group and 6 were from the SOC group. Examination of the characteristics of these patients did not provide any explanation for the difference in dropout rates between the two groups, and there is no evidence that treatment allocation played any role." (p.783) Reviewer note: There is insufficient information regarding reasons for missing outcome data and, therefore, to conclude that the reasons are unrelated to outcome. There are differences in the proportion of missing outcome data between intervention groups. There are no reasons for missing outcome data other than 'loss‐to‐follow‐up,' so uncertain as to whether these reasons differ by group. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol available. Published abstract (2014) listed the same primary outcomes, time points. Methods section indicates that one, commonly‐employed threshold adherence definition was planned; this outcome was reported in Results. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Olsen 2012.
| Methods | Randomised parallel‐group study | |
| Participants |
N = 100 with OSA diagnosed by PSG. Inclusion criteria: OSA confirmed by polysomnography, age ≥ 18, naive to CPAP Exclusion criteria: need for bi‐level ventilation, failed to complete CPAP titration, severe depression Baseline characteristics: 31% female (41.5% intervention, 28.3% control). Mean age 56.6 (±11.0). Mean RDI 34.3. Mean ESS 21.9. Mean BMI 34.5 Country: Australia |
|
| Interventions | Participants were randomised to motivational interviewing intervention (MINT, n = 53) or control (n = 53) group. MINT: three sessions of CPAP‐specific Motivational Interview Nurse Therapy (MINT) one month apart. Each session lasted approximately 30 minutes. In addition, all participants received standard one‐on‐one 45‐minute education session conducted on the day of CPAP titration. Participants were followed up at two to four weeks by physician and at two months by a nurse. A questionnaire and a machine meter data on adherence were obtained at one, three and 12 months Control: standard one‐on‐one 45‐minute education session conducted on the day of CPAP titration. Participants were followed up at two to four weeks by physician and at two months by a nurse Study duration: 52 weeks |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using envelopes with group allocation; no blocking or stratification used |
| Allocation concealment (selection bias) | Low risk | "...opaque, unlabelled envelopes...shuffled by a research assistant...placed into an allocation box held in a secured clinic area." Administrative officers not otherwise involved in the study withdrew an envelope and booked the participant's future appointments accordingly |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and intervention nurses were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The adherence analyses were by intent‐to‐treat...The multiple imputation method for substitution missing data was used...All univariate and bivariate statistical assumptions were met" |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | Low risk | Authors report, "An equal number of allocations for the control (n=53) and MINT (n=53) groups were placed into opaque, unlabelled envelopes, and the envelopes were shuffled by a research assistant. No blocking or stratification of randomisation was used. The envelopes were then placed into an allocation box held in a secure clinic area. After recruitment into the study, participants were directed to the sleep centre's administrative officers to schedule future appointments. The officer withdrew an envelope containing the intervention allocation and booked the patient's future appointments on that basis. The administrative officers were not otherwise involved in recruitment or provision of the intervention." Key baseline characteristics (age, gender, BMI, AHI) were reported for all randomised participants and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Authors report, "The nature of the intervention meant that we were unable to mask either the participants or the intervention nurses to treatment assignment." No deviations documented; none suspected based upon review. mITT Under Methods (Participants), authors report, "Participants were eligible for inclusion if they were at least 18 years of age, had a diagnosis of OSA confirmed by clinical polysomnography (PSG), had a clinical recommendation for CPAP treatment, and were naive to CPAP treatment. Participants were excluded from the study if they were found to require bi‐level ventilation (e.g. due to evidence of central sleep apnoea syndrome), did not complete a CPAP titration study, or were unable to give informed consent." Under Methods (Randomisation and Masking), authors report, "Eligible participants were randomly assigned...," indicating N = 53 for each intervention arm. Participants were booked for CPAP titration after allocation. Even though the cited reasons for post‐randomization exclusions would, in theory, have been determined following diagnostic PSG and, therefore, prior to randomisation, the reasons for post‐randomisation exclusions appear to have been due to eligibility being confirmed after randomisation. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Outcome data available for 94 of 106 (88.7%) randomised participants. However, 94% of eligible randomised participants have outcome data. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Unclear risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring. Unable to confirm with study author that distribution of CPAP device makes did not differ between intervention arms. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol available. Published abstract (2009) and published report (2012) listed the same primary outcomes, : "Objective CPAP adherence was assessed at 1, 2 and 3 months," except 12 month endpoint was included in final report. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned; each planned outcome reported. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Parthasarathy 2013.
| Methods | Randomised parallel‐group open‐label | |
| Participants |
N = 39 veterans with OSA prescribed CPAP. Inclusion criteria: age 21‐85, new diagnosis of OSA, AHI > 5, full night or split night polysomnography, no sedative medications used Exclusion criteria: central or complex sleep apnoea, requirement of oxygen or Bi‐PAP, unstable medical co‐morbidities, irregular lifestyle pattern, excess alcohol use Baseline characteristics: 0% female. Mean age 52 (±14). Mean AHI 37. Mean ESS 10.8. Mean BMI 34. Country: USA |
|
| Interventions | Participants were randomised to usual care (UC, n = 17) or peer buddy system (PBS, n = 22) group. PBS: trained peers with OSA and good CPAP adherence record were paired with newly diagnosed participants over three months. During two face‐to‐face sessions and eight telephone‐based conversations, trained peers shared their experiences on coping strategies with CPAP, knowledge of perceived vulnerabilities of untreated OSA, motivated participants and promoted methods for improving efficacy of CPAP UC: CPAP initiation and education class, participants were asked to send CPAP adherence 'smart cards' and were followed up at one and three months Study duration: 90 days |
|
| Outcomes |
|
|
| Notes | Additional information on study methods and mean CPAP adherence obtained from the study author. These data were available from a pilot study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was accomplished by computer‐generated assignment placed in sealed envelopes that were opened in a predetermined sequence of numbered and sealed envelopes |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Observers who evaluated outcomes and care providers were blinded to group allocation. Participants were not blinded to the intervention and were aware of CPAP adherence monitoring |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two of 17 participants in the control group lost to follow‐up versus zero in the intervention group No information on how this attrition was dealt with |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within METHODS: "This prospective, randomised, parallel group, open label, pilot study randomly assigned patients with OSA who had not yet been initiated on CPAP therapy to the peer‐buddy system to promote adherence to CPAP therapy (peer‐driven intervention group) or be provided with educational brochures regarding OSA and CPAP therapy (usual care group)." Study flow chart shows that baseline measurements were obtained on 39 participants and arrows indicate the numbers allocated to each intervention arm. Authors state this is a randomised study so the flow chart implies randomisation occurred after 'CPAP initiation and education by respiratory therapist. Authors do explicitly describe randomisation procedures or timing of randomisation, but it appears that key baseline characteristics (age, gender, BMI, AHI, ESS) were reported for randomised participants. The differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | open‐label No deviations documented; none suspected based upon review. mITT. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Authors report 2 participants (both in usual care group) were lost to follow‐up. Outcome measures table (which does not include CPAP usage at 90 days) is based on total randomised participants in each group (i.e. n = 22, n = 17). However, the authors do not report on CPAP usage at 90‐days (the pre‐specified outcome endpoint) in the text or table. They present a graph of minutes CPAP usage in each group by week and report the MANOVA results for comparison of repeated measures between groups. Authors do not explicitly report the Ns upon which these graphical data are based, but it is likely that it is either all randomised participants (n = 39) or all participants who were not lost‐to‐follow‐up (n = 37). Therefore, the primary CPAP usage/adherence data were available (to authors) for all or nearly all (95%) participants. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Unclear risk | Outcome measured using objective CPAP usage data. Outcome measured using objective CPAP usage data. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | High risk | Publication (2013), earlier abstract (2012) and ClinicalTrials.gov entry (first submitted 15 Jun 2010, last updated 7 Apr 2015) available and reviewed. Primary and secondary outcomes (submitted 15 July 2010) and are the same as current primary and secondary outcomes (i.e. no updates/changes were submitted to ClinicalTrials.gov by authors). No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. In Methods section of published report, authors indicate the following planned outcome measurement: "CPAP adherence downloads: Mean number of hours per day of CPAP use was collected for the entire 90 days through downloads on days 30 and 90." In results, outcome data for each week (1‐13) presented in graphical form. Only week 1 (not a pre‐specified outcome endpoint) described in the text. No other numerical CPAP usage results presented in tables or text. 90‐day endpoint result obtained from author. Thus, while authors seemed to have selected a different endpoint to be highlighted in the published report results section from the intended primary endpoint, the result we have assessed in our review (and obtained directly from authors) appears to have been pre‐specified at the primary outcome. Report presents a threshold‐based categorical adherence outcome not pre‐specified in the NCT entry (protocol) nor in the published report methods section. Per NCT entry (first submitted 15 June 2010), authors planned secondary outcomes (submitted 15 jJuly 2010) included 'CPAP adherence [time frame: three months]'. The abstract and published report indicate that CPAP usage/adherence data were collected over the full 90 days and were analysed over that time period. The published report and earlier abstract (2012) report MANOVA results for comparison of CPAP usage in each group by week and the published report presents a graphical representation of CPAP usage by week. However, neither the abstract nor the full published report contains numeric (minutes/day) results for the 90‐day pre‐specified outcome endpoint. The report only presents actual CPAP usage values (minutes/day) at 1 week and reports a threshold‐based categorical adherence outcome (proportion of participants in each group using at least 4 hours/night) at an unspecified time point. Neither of these outcomes appeared to be pre‐specified according to NCT entry, abstract and Methods section of published report. No other numerical CPAP usage results presented in tables or text. Nonethless, the numerical result being assessed for our review was for the pre‐specified CPAP adherence endpoint. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Pengo 2018.
| Methods | Randomised, parallel‐group study. | |
| Participants |
N = 112 patients who had positive home‐based pulse oximeter screen for OSA. Inclusion criteria: following at‐home screening using nocturnal pulse oximetry, patients who had 4% ODI ≥ 5 and typical symptoms of sleep apnoea (ESS > 10 points), or a 4% ODI > 15 were invited for CPAP treatment. Exclusion criteria: mental or physical disability precluding compliance with study protocol, unable to participate in trial follow‐up. Baseline characteristics: 25% female. Mean age 49.1 (±12.1). Mean ODI = 24.8. Mean ESS=11.3. Mean BMI = 36.5. Country: UK |
|
| Interventions | Participants were randomised to receive, in addition to CPAP therapy, either positively (n = 36) or negatively framed (n = 37) messages, or standard care (n = 39) alone. All patients received 2 weeks of APAP, followed by 4 weeks of fixed CPAP. Standard care: included explanation of importance of treating OSA, APAP introduction by expert sleep technicians, standard instructions on use of devices, review for troubleshooting, compliance assessment at 2‐weeks post treatment initiation. Positive: positively‐framed messages in addition to CPAP. Patients were phoned weekly and read the framed health messages (up to a total of 6 phone calls per patient). Negative: negatively‐framed messages in addition to CPAP. Patients were phoned weekly and read the framed health messages (up to a total of 6 phone calls per patient). Study duration: 6 weeks |
|
| Outcomes |
All outcomes reported at 2 and 6* weeks. |
|
| Notes | Intervention arms (positively‐ and negatively‐valenced messages) combined for comparison to control arm in meta‐analysis, as recommended in Cochrane Handbook section 16.5.4. * Indicates primary outcome analysed in this Review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No reference to how randomisation was achieved. |
| Allocation concealment (selection bias) | Unclear risk | No reference to allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No evidence of blinding of participants, personnel or outcome assessors. Study only had objective outcomes, so lack of blinding is not likely affect outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data missing for 24 of the 112 (21%) randomised. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Published abstract (2014) listed the same primary outcomes. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalized before unblinded outcome data were available for analysis. |
| Other bias | Low risk | No baseline imbalances or reported/suspected deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Authors report: "Patients were randomly assigned to one of the three groups: the first received positively framed messages in addition to CPAP, the second received negatively framed messages in addition to CPAP, and a control group entailed patients who received best standard care with CPAP, but no framed messages. The three groups were matched for age, sex and BMI." No reference to random component or allocation concealment method. Key baseline characteristics (age, gender, BMI, AHI) were reported for all randomised participants and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. Likely mITT. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | Outcome data missing for 24 of the 112 (21%) randomised. Neither analyses to correct for bias nor sensitivity analyses were conducted. Loss to follow‐up could be related to participant health status. There were differences in the proportions of missing outcome data among intervention groups: intervention group 1 (5/36) intervention group 2 (8/37) and control group (11/36). Authors note "p<0.05 for comparison between positively framed and control group," and that reasons for loss‐to‐follow‐up were either the participant returned the CPAP machine or could not be contacted. They do not specify reasons by intervention group. Returning the machine and not responding to contact attempts are both potentially related to the true value of the outcome (i.e. those who returned their machines early or did not respond to calls may be less compliant than those who continue in the study). Favours comparator. If loss to follow‐up associated with reduced adherence, control group would have lower adherence and advantage of experimental intervention would be underestimated. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol available. Published abstract (2014) listed the same primary outcomes. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned; each planned outcome reported. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Pepin 2019.
| Methods | Randomised, multi‐centre parallel‐group study. | |
| Participants |
N = 306 patients with newly‐diagnosed OSA . Inclusion criteria: 18 to 75 years, severe OSA (AHI > 30) on the basis of respiratory polygraphy or PSG, at least one cardiovascular disease or exhibit an elevated cardiovascular risk (Systematic Coronary Risk evaluation risk > 5% at 10 years or in secondary prevention). Exclusion criteria: CSA, heart failure with a left ventricular ejection fraction < 40%. Baseline characteristics: 26% female. Median age 61.3 (IQR: 54.1‐66.1). Median AHI = 46. Median ESS = 9. Median BM I =32.0. Country: France |
|
| Interventions | Participants were randomised to usual care (UC, n = 149) or multimodal telemonitoring (TM, n = 157) for 6 months. TM: CPAP‐related factors (adherence, leaks, and residual events), BP and physical activity recorded by connected devices. Symptoms and quality of life were recorded via electronic questionnaires completed by patients. Patients received demonstration home telemonitoring use and an explanation of why monitoring these physiological variables was relevant for their care. Automatic algorithms were constructed for the prompt adjustment of CPAP treatment. UC: Received standard care usually received from their assigned sleep centres. Study duration: 6 months |
|
| Outcomes |
All outcomes were reported for 6 month endpoint only. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to multimodal remote tele‐monitoring or usual care (1:1 ratio) using computer‐generated allocation. Randomisation was stratified by centre, home care provider, and CPAP brand |
| Allocation concealment (selection bias) | Unclear risk | No reference to allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At baseline, 306 patients were randomised: 157 to telemonitoring and 149 to usual care. 40 of 157 (18.1%) and 27 of 149 (25.5%) were reported as 'lost during follow‐up' in the treatment and control arm, respectively. |
| Selective reporting (reporting bias) | Low risk | NCT information first submitted 15 Feb 2013, last updated 5 Sep 2014. Study start date reported as Feb 2013. Primary outcome (change from baseline CPAP compliance at 6 months) submitted 20 Feb 2013 and is the same as current primary outcome (i.e. no updates/changes were submitted to ClinicalTrials.gov by authors). Published report specifies the same primary outcome as that specified in NCT entry. Dates provided indicate that analysis plan was finalised before unblinded outcome data would have been available for analysis (i.e. outcome specified same month as recruitment period started). |
| Other bias | Low risk | No baseline imbalances or reported/suspected deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Authors report, "Participants were randomised to multimodal remote tele‐monitoring or usual care (1:1 ratio) using computer‐generated allocation. Randomization was stratified by centre, home care provider, and CPAP brand (four main brands used in France)." No reference to allocation concealment method. Key baseline characteristics (age, gender, BMI, AHI) were reported for all randomised participants and differences appeared non‐significant, but no P values reported. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Open‐label. Authors report, "Neither participants nor investigators were masked to group assignment." No documented deviations suspected to have arisen because of experimental context.Authors report, "Data were initially analysed in intention‐to‐treat (ITT) including all randomised patients; then, a per‐protocol analysis was done on all randomised participants excluding study dropouts. To replace missing data, multiple imputations were performed for the primary outcomes by using a logistic and linear regression model for binary or continuous variables, respectively. Fifty data sets were constituted." Documented deviations from protocol (not expected to have arisen because of experimental context: "The main limitation of this study was the failure to reach the estimated sample size. During the course of the study, reimbursement for telemonitoring was suspended in France because of opposition to it by several patients' associations. For the first time, the French health‐care authorities had attempted, by decree, to make coverage of health costs conditional on patient's adherence to treatment. Patients successfully appealed against this decree through the highest court "Conseil d'Etat." Consequently, the trial data monitoring committee recommended trial termination for pragmatic reasons...." |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | At baseline, 306 patients were randomised: 157 to telemonitoring and 149 to usual care. 40 of 157 (18.1%) and 27 of 149 (25.5%) were reported as 'lost during follow‐up' in the treatment and control arm, respectively. Difference in proportion (N‐1 chi‐square test: chi‐sq=2.455, DF=1, P = 0.1171; z‐test: z=‐1.5696, P = 0.1164) is not statistically significant. Neither analyses to correct for bias nor sensitivity analyses were conducted. Missing outcome data occurred due to loss to follow‐up, which could be related to the outcome. Therefore, it is possible that missingness in the outcome was influenced by its true value. There are differences in the proportion of missing outcome data between intervention groups (see 3.1), but these are non‐significant: N‐1 chi‐square test: difference=7.4% (95%CI: ‐1.86‐16.6%), chi‐sq=2.455, DF=1, P = 0.1171. There are no specific reasons for missing outcome data other than 'lost during follow‐up,' so cannot determine if reasons differ between study arms. Per Cochrane handbook, 8.13.2.2, "Even if incomplete outcome data are balanced in numbers across groups, bias can be introduced if the reasons for missing outcomes differ." Without specific reasons for missing data, the potential for bias cannot be adequately assessed. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring. Randomisation was stratified by centre, home care provider, and CPAP brand. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | Publication (2019), abstract (2017) and ClinicalTrials.gov entry, NCT01796769 available and reviewed. NCT information first submitted 15 Feb 2013, last updated 5 Sep 2014. Study start date reported as Feb 2013. Primary outcome (change from baseline CPAP compliance at 6 months) submitted 20 Feb 2013 and is the same as current primary outcome (i.e. no updates/changes were submitted to ClinicalTrials.gov by authors). Published report specifies the same primary outcome as that specified in NCT entry. Actual primary completion date was listed in NCT entry as: 'Sep 2014 (final data collection for primary outcome measure)'. Recruitment period specified in published report was Feb 2013 to Oct 2013. Dates provided indicate that analysis plan was finalised before unblinded outcome data would have been available for analysis (i.e. outcome specified same month as recruitment period started). Methods section indicates one outcome time point (for primary adherence outcome) was planned. Results section reports one outcome time point. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Richards 2007.
| Methods | Randomised, parallel‐group trial | |
| Participants |
N = 100 participants with newly‐diagnosed OSA referred for CPAP titration Inclusion criteria: newly diagnosed with OSA referred for CPAP titration Exclusion criteria: inability to understand fluent English, previous use of CPAP. Baseline characteristics: 4% female. Mean age 56. Mean RDI 26.5. Mean ESS 10.5. Mean BMI 30.3. Country: Australia |
|
| Interventions | Participants were randomised to treatment as usual (TAU, n = 50) or Intervention (n = 50) group. Intervention: CBT. Two one‐hour group sessions; slide presentation on sleep, OSA and treatment. CPAP machine on display and relaxation techniques in the event of anxiety caused by wearing CPAP mask Participants also benefited from video presentation with emphasis on perseverance with treatment and educational pamphlet made available TAU: one standardised group education session; explanation of CPAP titration process; familiarisation with equipment used and procedure to be followed on the titration night. Explanation of side effects, all participants strongly encouraged to contact staff to obtain relevant help and support. Participants assessed and fitted with comfortable mask to be worn during titration Study duration: 28 days |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...a sequence generated with a blocking factor of 4" |
| Allocation concealment (selection bias) | Low risk | "An investigator not involved with recruitment or provision of treatment independently randomised participants using a sequence generated with a blocking factor of 4. Allocation concealment was achieved with sequentially numbered, opaque, sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not possible/attempted for participants; assessors and technicians not informed of treatment groups "Staff members were blinded to which group participants had been allocated and the 3 usual CPAP therapists strictly adhered to a script" Participants not informed that machine usage would be monitored |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High attrition rate in control group (17/48 refused to take CPAP home) "Analysis was by intention to treat, and we measured hours of usage of CPAP at 28 days" |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | Low risk | Authors report: "An investigator not involved with recruitment or provision of treatment independently randomised participants using a sequence generated with a blocking factor of 4. Allocation concealment was achieved with sequentially numbered, opaque, sealed envelopes. Of the 109 individuals approached, 9 refused to participate. Consenting participants were randomly assigned to either a treatment as usual group (TAU) (n = 50) or to the CBT group (n = 50)." Random component not specified (how random sequence generated). Allocation concealment method specified and appropriate, but block size not specified. Key baseline characteristics (age, gender, BMI, AHI) were reported and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Report does not specify which staff members were blinded and which were involved in delivering the intervention. No deviations documented; none suspected based upon review. Authors report in stats section that analyses were ITT. However, flowchart indicates "Usage assessed at day 28 (n=48)." for each tx group. Additionally, under results section, authors write: "study. After attending the CPAP‐pressure determination study, 2 participants were withdrawn from the study. One participant allocated to CBT was found to require bilevel noninvasive ventilation, and a participant allocated to treatment as usual went overseas for an indefinite period of time. The data from these participants were excluded from the outcome analysis at 7 and 28 days. The Smart‐Card data from 2 participants at 28 days was lost in the mail, and these data were also excluded from this part of the analysis." This suggests analysis was mITT. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | 96 of 100 had outcome data at endpoint day 28. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the METHODS section of the publication. Methods section indicates that multiple time points planned; each planned outcome reported. Methods section indicates that multiple threshold‐based adherence analyses were planned and all were reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | Concerns primarily derive from lack of availability of a protocol or pre‐specified analysis plan, which is common for older studies. |
Roecklein 2010.
| Methods | Randomised parallel‐group study | |
| Participants |
N = 30 patients diagnosed with OSA by PSG, naive to CPAP and reporting intent to use CPAP. Inclusion criteria: age 18 to 65, CPAP naive, reported intent to use CPAP (other sleep, psychiatric or health problems were not exclusion criteria) Exclusion criteria: none reported. Baseline characteristics: 70% female. Mean age 46.3 (±11.2). Mean AHI 44.4. Mean ESS 11.6. Mean BMI 42.1. Country: USA |
|
| Interventions | Participants were randomised to standard education (SE, n = 16) or personalised feedback (PF, n = 14) group. PF: written personalised feedback report, including detailed information on severity of the disease, self‐reported daytime sleepiness, individually estimated risk of adverse health outcome and risk of motor vehicle accident, all compared with normative data. Feedback addressed barriers to using CPAP, ambivalence about treatment and difficulties of behaviour change and promoted self‐efficacy and personal responsibility for choosing to use CPAP SE (control): written information from the American Academy of Sleep Medicine on OSA, Snoring and PAP therapy for OSA Study duration: 3 months |
|
| Outcomes |
|
|
| Notes | Participants were not provided machines but obtained them 'naturalistically', most commonly through insurance. Most participants were low‐income African Americans | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Physicians were blind to study participation and participants were blind to their study condition." Patients were aware that CPAP usage was monitored. Despite intended blinding, it is likely that participants would have been able to distinguish the two interventions |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only two incidents of missing data in each group. However, in addition, participants who took longer to obtain machines (n = 5 in control group and n = 2 in intervention group did not obtain devices by two weeks) were included from the start and had CPAP usage recorded as 0 hours per session. It is possible that financial burden prevented some participants from acquiring CPAP machines in a timely fashion |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: "Consecutive clinic patients were enrolled after receiving their OSA diagnosis and CPAP prescription. Participants were randomly assigned to feedback or standard information groups." Key baseline characteristics (age, gender, BMI, AHI, ESS) were reported and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Authors report, "Physicians were blind to study participation, and participants were blind to their study condition." No information is provided as to who distributed the written intervention materials to the participants and how blinding was maintained from physicians or other study personnel. No deviations documented; none suspected based upon review. In Methods (Measures) section, authors report, "Individuals who had not obtained machines (2 weeks: control, n =5 and feedback, n=2; 3 months: control, n =2 and feedback n =1) were recorded as having used CPAP for 0 hours per sessions. Individuals whose machines did not record use or who forgot to bring machines to the research session were treated as missing data (2 weeks: control, n = 1 and feedback, n= 0; 3 months: control, n =2 and feedback n = 2). Groups did not differ in the rates of missing versus present data at 2 weeks:X2(2,n=27)=3:86, ns; or 3 months: X2(2,n= 28) = 0:86, ns." In results, authors report, "Participants were 14 in the feedback group and 16 in the control group, and each group lost 1 to follow up." As the difference in Ns implies that the 'lost‐to‐follow‐up' participants were neither those who did not obtain machines nor those for whom machines did not record use or who forgot to bring machines, it is not clear whether the lost‐to‐follow‐up were assigned 0 hours/session or were treated as missing and excluded from analysis. Results tables do not indicate the Ns upon which the recorded results are based. Most likely, the results are based on mITT analysis. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | Authors do not report the number of participants randomised and no Ns were reported in the results table. In Methods (Measures) section, authors report number in each group who did not obtain machines and in each group whose machines did not record use or who forgot to bring machines to research session. It is not clear if those who didn't get machines, whose machines did not record or who forgot to bring machines were amongst the participants referenced in the results section, "Participants were 14 in the feedback group and 16 in the control group, and each group lost 1 to follow‐up." Thus, it is not clear whether the 15 total participants (N = 5 in intervention arm , N = 10 in control arm) who had no machine or no outcome data were amongst the 30 participants (N = 14 intervention, N = 16 control) referenced in the results or were in addition to them. Therefore, the total randomised number could have been as low as 15 (6,9) or as high as 45 (19, 26), depending upon whether the participant numbers in results section were numbers before or after accounting for those who did not obtain machines or had missing data. It is also not clear whether the one per group who were lost to follow‐up were amongst or excluded from the reported N values. Neither analyses to correct for bias nor sensitivity analyses were conducted. Missing outcome data occurred for documented reasons, some of which ('forgot' CPAP device/data) could be related to the outcome. There were non‐significant differences between groups in proportion of participants who met authors' definition of missing outcome data (n = 2 for each group at month 3). Reasons cited for missing data were either 'machines did not record use or who forgot to bring machines to the research session.' The latter reason (i.e. 'forgetting') can be related to outcome while machine failure would not be. The authors did not distinguish amongst those reasons in reporting the numbers of participants with missing outcome data for each intervention group. Thus, it is possible that there were differences between groups in the proportions missing because of 'forgetting'. Comparison of possible proportions is further complicated by the fact that we do not have the actual randomised denominators for each group. It is possible that both intervention participants with missing data had 'forgotten' to bring it for analysis while those missing data in control group were purely based on chance equipment failure. Depending on respective denominators, under the above‐noted conditions, there would be a difference of 14% to 0% (intervention vs. control) or 10.5% to 0% (intervention vs. control) in proportion missing due to 'forgetting,' depending on actual randomised Ns. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring. Unable to confirm with study author that distribution of CPAP device makes did not differ between intervention arms. However, likely sufficiently similar (i.e. probably Respironics devices) based on authors note "95% of machines detected breathing; Respironics Inc., Murrysville, PA", suggesting most/all participants for whom data were available were using a Respironics device) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned; each planned outcome reported. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Sarac 2017.
| Methods | Randomised, parallel‐group study. | |
| Participants |
N = 115 patients with OSA. Inclusion criteria (not explicit): ≥ 18 years old), newly diagnosed OSA (AHI ≥5), free from upper airway obstructions. Exclusion criteria (not explicit): not interested in PAP or in study participation, living outside Istanbul, unable to come to follow‐up. Baseline characteristics: 24.5% female. Mean age 51 (±9.3). Mean AHI = 41.4. Mean ESS =10.0. Mean BMI = 32.5. Country: Turkey |
|
| Interventions | Participants were randomised to receive standard support (SS, n = 63) or educational support (ES, n = 52). SS: general explanation (˜10‐15 minutes) of OSA and PAP. ES: SS + additional education (˜ 20 minutes) by a sleep medicine physician , including: viewing his/her own polysomnography chart on morning post PAP‐titration, comparing the PSG from diagnostic and CPAP titration studies with explanations that emphasised obstructive events and oxygen desaturations, and the disappearance of those signs on PAP treatment. Study duration: approximately 6 months |
|
| Outcomes |
|
|
| Notes | *58 out of 63 patients in the SS group, and 49 out of 52 patients in the ES group completed the five follow‐up appointments during the study period. The median time from randomisation to first follow‐up was 20 days for both groups with an IQR 17–27 days for the SS group, and 16–26 days for the ES group (P = 0.89). The median time to last follow‐up was 187 days (IQR 170‐202 days) in the SS group, and 184 days (IQR 173–198 days) in the ES group (P = 0.16). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomly assigned in order of appearance (random number table: SS/ES) with exception for patients scheduled for weekend treatment, who were included in the SS group." |
| Allocation concealment (selection bias) | High risk | Sequence not concealed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No evidence of blinding of participants, personnel or outcome assessors. Study only had objective outcomes, so lack of blinding is not likely affect outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/63 and 3/52 participants were lost to follow‐up (never used device+did not come to final visit) in SC and INT groups respectively, less than 10% |
| Selective reporting (reporting bias) | Unclear risk | No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. |
| Other bias | Low risk | Baseline differences were insignificant for all variable s of interest. Although there was a deviation from the intervention (phone calls to reinforce use of machine), this was done for all participants regardless of group assignment. |
| Bias arising from the randomisation process (ROB2, primary outcome) | High risk | Authors report, "The remaining 115 patients were randomly 1: 1 assigned to standard support (SS) group (general information about OSA and PAP treatment at baseline), or to educational support (ES) group (additional polysomnography chart viewing from both diagnostic and titration nights). Participants were randomly assigned in order of appearance (random number table: SS/ES) with exception for patients scheduled for weekend treatment, who were included in the SS group." Key baseline characteristics (age, gender, BMI, AHI) were reported and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Unclear risk | No blinding. Authors report, "... the patients not coming to the scheduled follow‐up visit were contacted by phone by the sleep physician, which might have reinforced the patients participation. This might be considered a bias. However, the phone calls were done for all participants regardless of the group allocation." It is not clear that phone call reminders were an intended aspect of the protocol or added later (i.e. a deviation). ITT. Authors report, "Two patients in the SS group, and two patients in the ES group never used their devices, and their CPAP usage hours/night was included in the final analysis as 0." |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Authors report, "Two patients in the SS group, and two patients in the ES group never used their devices...." Thus, outcome data were available for 96.5% of randomised participants. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. This study permitted use of CPAP, APAP and BiPAP; however, distribution comparable across intervention arms as per authors, "Distrubution of CPAP, APAP, and BPAP devices was 71.4%, 4.8%, and 23.8%, respectively, in the SS group, and 71.2%, 7.7%, and 21.2%, respectively, in the ES group (p=0.65)." Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | ClinicalTrials.gov entry (NCT02756299) indicates that the current primary outcome was unchanged from original primary outcome measure, "Positive Airway Pressure usage (hours/night) (Time Frame: 6 months) ‐ Satisfactory device usage defined as minimum 4 hours of night during at least 70% of period based on the objective measures from the device." (submitted 26 Apr 2016). Final data collection date for primary outcome was April 2015.
In published report, the primary outcome endpoint was defined only as 'at the last visit' and time of this 'long‐term compliance' outcome varied across participants.
No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Given that primary endpoints not defined and outcomes assessed at 5 time points, insufficient information to determine if 'short‐term' and 'long‐term' endpoint definitions were prespecified. Short‐term compliance outcome not mentioned in NCT entry. Methods section indicates that one, commonly‐employed threshold adherence definition was planned; this outcome was reported in Results. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Sawyer 2017.
| Methods | Randomised, parallel‐group study. | |
| Participants |
N = 118 adults with newly diagnosed OSA Any adult patient referred for a diagnostic PSG was invited to participate in the study. Inclusion criteria: newly diagnosed with OSA (AHI > 10), PAP‐naive, ≥18 years of age, able to read and speak English. Exclusion criteria: previous diagnosis or treatment of OSA; medical record documented new psychiatric diagnosis within previous six months of study enrolment; requirement of supplemental oxygen or bilevel PAP identified on PAP titration PSG suggesting diagnosis other than OSA; diagnosis of another sleep disorder in addition to OSA based on polysomnogram (i.e. periodic limb movement disorder [≥10 limb movements/hr of sleep with arousal], central sleep apnoea [≥ 5/hours central apneas], insomnia, sleep hypoventilation syndrome, or narcolepsy). Baseline characteristics (per‐protocol): 30% female. Mean age 51.3 (±11.1). Mean AHI = 36. Mean ES S= 19.6. Mean BMI=38.0. Country: USA |
|
| Interventions | Participants were randomised to receive usual care (UC, n = 57) or a multi‐phased and tailored intervention (TI, n = 61) targeting social cognitive perceptions of OSA–PAP treatment. TI: intervention addressed cognitive perceptions of the diagnosis and treatment, outcome expectancies with PAP treatment, and PAP treatment self‐efficacy, all domains of SCT. Intervention delivered in four phases: prediagnosis, postdiagnosis (i.e. postdiagnostic polysomnogram), immediately post‐PAP titration polysomnogram, and with week 1 of home PAP treatment. Intervention delivery guided by a protocol and script templates for specific exposure phases to minimise a potential interventionist effect. UC: followed current practice standards for the diagnosis and treatment of OSA in adults (Epstein 2009; Kushida 2006). Included sleep centre–provided informational brochures about OSA, diagnostic testing, and PAP prescription. In addition, access by telephone to sleep centre staff for problems, questions, or concerns was provided during daytime and evening. Study duration: 3 months |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation employing random block sizes assigned participants to the exposure or comparison group and within each assignment level, 50% were randomly assigned to interview–no interview at study termination and debriefing. A randomisation list was generated by the study biostatistician (TSK) and securely maintained at the clinical research site. |
| Allocation concealment (selection bias) | Low risk | Random assignment was concealed and completed by consecutive sealed envelope, then opened sequentially by interventionist and study research assistant; sealed envelopes were secured, prepared, and monitored by unblinded study personnel |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No evidence of blinding of participants, personnel or outcome assessors. Study only had objective outcomes, so lack of blinding is not likely affect outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data not available for participants excluded (intentionally) post‐randomisation. Reported reasons for missing outcome data may be related to health status. |
| Selective reporting (reporting bias) | Low risk | Published report Methods provides a comprehensive description of the intervention design, intervention protocol, study design, setting and sample, measures and analysis. Additionally, the pre‐specified plan included per‐protocol fidelity measures and a blinding assessment for participants upon study completion. |
| Other bias | Low risk | No baseline imbalances or reported/suspected deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Low risk | Authors report: "After pre enrolment screening and informed consent, participants completed a demographic questionnaire and were randomised. Randomization employing random block sizes assigned participants to the exposure or comparison group and within each assignment level, 50% were randomly assigned to interview–no interview at study termination and debriefing. A randomisation list was generated by the study biostatistician (TSK) and securely maintained at the clinical research site. Random assignment was concealed and completed by consecutive sealed envelope, then opened sequentially by interventionist and study research assistant; sealed envelopes were secured, prepared, and monitored by unblinded study personnel (DAS)." Key baseline characteristics (age, gender, BMI, AHI) were reported and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Authors report, "In order that a participant blind was supported from the outset of the trial, IRB‐approved consent modification was employed that specifically did not differentiate between study groups in terms of study activities or time commitment and a limited description of the overall study objective; this necessitated debriefing at study termination."
Regarding investigator blinding, authors report: "The intervention was delivered by one research assistant (study interventionist, unblinded, MV), a registered nurse without sleep specialty care experience, who was extensively trained to provide the tailored intervention." participants were randomised prior to PSG; therefore, some of the inclusion criteria could only be assessed after randomisation. Of the 118 randomised participants, 30 (18 in intervention arm and 12 in control arm) were excluded based on failure to meet AHI = 10 inclusion criteria. Additionally, authors report: "Exclusions or withdrawals occurred for the following reasons: refused PAP (n = 2), referred to other treatment (n = 1), and titrated on other positive airway device for other sleep‐related breathing disorders (n = 10). Specific to the protocol, four participants did not complete titration PSG and eight participants requested to withdraw due to personal (i.e. transportation, familial issues, work‐related issues) or other pressing health problems. The remaining participants (n = 60) completed the protocol; no attrition or loss of data for the PAP use outcome or feasibility and acceptability outcomes occurred. No study‐related adverse or serious adverse events occurred." Non‐OSA or BiPAP‐requiring sleep disorders were planned exclusionary criteria; these exclusions should not be considered protocol deviations. Additionally, CPAP refusal and referral to other treatment (by provider) are also not deviations from intended interventions in this study. Thus, consistent with author's report, only 12 protocol deviations (6 in each arm) occurred. Administrative withdrawal: 2/31, 2/27; subject withdrawal: 4/31 and 4/27. mITT. Outcome data were not available for participants who withdrew (n = 12). |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Unclear risk | Data not available for participants excluded (intentionally) post‐randomisation. Neither analyses to correct for bias nor sensitivity analyses were conducted. It is possible that withdrawals were related to health status, as per the reasons outlined in the report (transportation, familial issues, work‐related issues, pressing health problems). The proportion of missing outcome data is similar across groups. Reported reasons make it possible that missingness is related to its true value. However, reported reasons do not differ between groups. While continuing symptoms may have made it more likely for participants to drop out, the roughly equivalent withdrawal rates across groups makes it unlikely that this would have resulted in substantial differences in the estimated effect of the intervention. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Unclear risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring. Outcome "assessor" is CPAP device: no knowledge of allocation possible. Unable to confirm with study author that distribution of CPAP device makes did not differ between intervention arms. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Low risk | Published report Methods provides a comprehensive description of the intervention design, intervention protocol, study design, setting and sample, measures and analysis. Additionally, the pre‐specified plan included per‐protocol fidelity measures and a blinding assessment for participants upon study completion. Methods section indicates that multiple time points planned; each planned outcome reported. Methods section indicates that one, commonly‐employed threshold adherence definition was planned; this outcome was reported in Results. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Scala 2012.
| Methods | Randomised, parallel‐group study. | |
| Participants |
N = 28 patients with newly‐diagnosed OSAS. Inclusion criteria: newly‐diagnosed, OSAS. Exclusion criteria: not reported. Baseline characteristics: 75.3% female.Mean age 57 (±11.2). Mean AHI NR. Mean ESS 12.6. Mean BMI NR. Country: Italy |
|
| Interventions | Participants were randomised to standard care (SC, n = 15) or an educational intervention (EDU, n = 13). EDU: 3 interactive sessions, video with discussion, focus group and role play, respectively 1, 2 and 3 months after receiving the CPAP device. Study duration: 6 months |
|
| Outcomes |
Outcomes measured at 6 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided regarding randomisation or how randomisation was achieved. |
| Allocation concealment (selection bias) | Unclear risk | No information related to allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information available regarding missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. |
| Other bias | Unclear risk | No information present to determine baseline imbalances or deviations from intended interventions. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Based on information provided in translator form. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Authors report analyses were conducted by intention to treat. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Unclear risk | Based on information provided in translator form. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring. We confirmed by personal correspondence with author that all participants received the same CPAP device make and pressure delivery system. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned. One endpoint (6‐month) reported in the published study and one (12‐month) noted to be 'in progress.' No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Sedkaoui 2015.
| Methods | Randomised, parallel‐group study. | |
| Participants |
N = 379 with newly diagnosed SAHS Inclusion criteria: SAHS, prescribed CPAP, AHI ≥ 30 or AHI < 30 and > 10 arousals/hour, French fluency. Exclusion criteria: age <18 years, under guardianship, previous CPAP use, psychiatric illness, participating in another clinical trial Baseline characteristics: 72.0% female. Mean age 63. Mean AHI 42.2. Mean ESS 11.6. Mean BMI 40. Country: France |
|
| Interventions | Participants were randomised to standard support (SS, n = 190) or coached support (CS, n = 189). SS: received information from their physician about modalities and usefulness of CPAP treatment. Technician performed CPAP set‐up at participant's home, re‐explained the device's function, and checked for mask fit and adaptation. Follow‐up performed at 1 month and 4 months to assess CPAP parameters. CS: in addition to SS, participants in CS received standardised support completed through 5 sessions (day 3 , 10, 30, 60, and 90) via telephone‐base counselling. Session 1 objective was to assess patient's knowledge about the disease, device and health consequences; to emphasise importance of good adherence; to encourage CPAP use throughout sleep every day. Objectives of the other educational sessions were to identify disadvantages or obstacles CPAP treatment and then focus on the benefits linked to use of CPAP. A particular effort was made to discuss misconceptions about sleep apnoea and barriers to use, concerns fears and beliefs, as well as the perceptions of their partners and family, in order to increase patients' positive expectations regarding CPAP benefits. Study duration: 4 months |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization of patients was performed 1/1 ratio at each individual centre, by an automatic computer system." No reference to specific random component (e.g. random numbers generated/assigned by computer). |
| Allocation concealment (selection bias) | Unclear risk | No reference to allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No evidence of blinding of participants, personnel or outcome assessors. Study only had objective outcomes, so lack of blinding is not likely affect outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 57 participants discontinued from the educational intervention, however, only data related to adherence was collected (and authors state that even though the intervention was discontinued, compliance was still measured for all participants). |
| Selective reporting (reporting bias) | High risk | Considerable evidence demonstrating that CPAP adherence time points were chosen after data was collected. The authors report, "In France, the condition for reimbursement by the national health system was a length of use of 3 or more hours per night over a 5 month period." The authors provide no justification for their choice to use a 4‐month study endpoint, which is not only atypical among CPAP adherence studies but also not consistent with their cited national health system definition. Since these data are registered by the CPAP devices continuously, any endpoint within the 6‐month collection period could have been used, including the one used by the national health system definition (5 months). Instead, only a 4‐month endpoint was reported. With no means of determining if the 4‐month primary outcome was specified prior to the availability of unblinded outcome data (NCT entry not made until after completion of primary data collection and very close to the time of final report publication), the available information suggests the possibility that the endpoint being assessed/reported by trialists was selected on the basis of results. |
| Other bias | Low risk | No baseline imbalances or reported/suspected deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: "Randomization of patients was performed 1/1 ratio at each individual centre, by an automatic computer system." No reference to specific random component (e.g. random numbers generated/assigned by computer) or allocation concealment method. Key baseline characteristics (age, gender, BMI, AHI) were reported for all randomised participants and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | NCT02435355: Listed as open‐label on ClinicalTrials.gov entry. Published report mentions neither blinding or masking. Authors report, "Not all patients with coaching received the 5 phone calls as prescribed in the procedure due to business activity, holidays or patient requests to stop the phone calls. 85 % of patients in CG received at least 4 phone calls and 70 % received 5 phone calls. In Fig. 5, we observed a significant and gradual link between patient phone calls received and mean hours of CPAP use." The protocol included that patients in coached group (intervention arm) receive 5 sessions of telephone‐based counselling. Thus, this was a protocol deviation. The reasons cited for the deviation, however, are likely not related to experimental context. Rather, they are consistent with routine care (i.e. scheduling issues, non‐adherence). Authors report, "Analysis was by intention to treat. Outcome data from patients randomised in the coached group were analysed even if they dropped out of the study or refused phone calls." CONSORT flow diagram indicates that analyses were performed on randomised participants. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Authors report, "Outcome data from patients randomised in the coached group were analysed even if they dropped out of the study or refused phone calls." In addition to information provided in CONSORT flow diagram, this suggests outcome data were available for all participants. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Unclear risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring. Unable to confirm with study author that distribution of CPAP device makes did not differ between intervention arms. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | High risk | Publication (2015), earlier abstract (2013) and ClinicalTrials.gov entry (first submitted 30 Apr 2015, last updated 06 May 2015) available and reviewed. Primary outcome submitted 05 May 2015 and is the same as current primary outcome (i.e. no updates/changes were submitted to ClinicalTrials.gov by authors). NCT entry and publication indicate participants were recruited from April 2010 to March 2012 and actual primary completion date was listed in NCT entry as: 'Aug 2012 (final data collection for primary outcome measure)'. There is insufficient information to determine whether analysis plan was finalised before unblinded outcome data were available for analysis particularly since information was posted to ClinicalTrials.gov in close proximity to publication of the final report (i.e. both in 2015). Although the protocol (NCT entry) and published study provide no direct evidence that multiple outcome endpoints/definitions were planned or evaluated, other information provided raises some concerns about the possibility of selective reporting. According to protocol (NCT entry) and methods section of the published report, the authors planned one threshold‐based adherence definition (> 3 hours per night for the first 4 months) and this was the only threshold‐based outcome reported in Results. The authors report, "In France, the condition for reimbursement by the national health system was a length of use of 3 or more hours per night over a 5 month period." Thus, although the 3‐hour threshold definition is not one that is commonly‐employed by other trialists in the literature, the use of this nightly threshold is reasonable for a study in France. On the other hand, the authors provide no justification for their choice to use a 4‐month study endpoint, which is not only atypical among CPAP adherence studies but also not consistent with their cited national health system definition. Furthermore, CPAP usage data was collected/downloaded at both a 3‐ and 6‐month visit. Since these data are registered by the CPAP devices continuously, any endpoint within the 6‐month collection period could have been used, including the one used by the national health system definition (5 months). Instead, only a 4‐month endpoint was reported. With no means of determining if the 4‐month primary outcome was specified prior to the availability of unblinded outcome data (NCT entry not made until after completion of primary data collection and very close to the time of final report publication), the available information suggests the possibility that the endpoint being assessed/reported by trialists was selected on the basis of results. Finally, the difference between intervention and control group is small (for both primary and secondary outcomes) and only barely reach statistical significance, selection bias could have influenced the reported effect estimates. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Shapiro 2017.
| Methods | Randomsied parallel‐group trial | |
| Participants |
N = 46 newly‐diagnosed with OSA and prescribed CPAP for the first time. Inclusion criteria: ≥ 18 years; newly‐diagnosed by PSG; commencing CPAP for first time; able to read/speak/understand/write English; CPAP with smart card technology Exclusion criteria: requires BiPAP, significant craniofacial abnormalities, Downs syndrome, cognitive delay, hypotonia, neuromuscular degenerative disorder, taking anti‐anxiety medication, pregnant. Baseline characteristics: 45.5% female, Mean age 51.8 (13.1). Mean AHI 26.2. Mean ESS NR. Mean BMI 35.7. Country: USA |
|
| Interventions | Participants were randomised to standard care (SC, n = 33) or CPAP‐SAVER Intervention (CI, n = 33). SC: basic OSA and CPAP teaching and follow‐up provided by respiratory therapist/CPAP education employed by home medical supplier. CI: Standard care plus airway model, video education sheet, report card components, support calls. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation procedures adequately described and were sufficient. |
| Allocation concealment (selection bias) | Unclear risk | There is insufficient information regarding allocation concealment to make a determination as to whether it was possible for enrolling investigators/staff to have knowledge of forthcoming allocation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Authors report participants were "masked as to group assignment," but do not describe the methods by which this masking was carried out for a behavioural intervention. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 of 33 (intervention) and 0 of 33 (control) were lost to attrition. |
| Selective reporting (reporting bias) | Low risk | This is a dissertation and the author presents a comprehensive protocol with design and analytic plan. Thus, it is likely that analytic plan was finalized before unblinded outcome data were available for analysis. |
| Other bias | Low risk | No baseline imbalances reported for variables of interest. Although there was a deviation from protocol (reduction of number of study sites) this seems to not have resulted in a deviation from the intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | After consent, participants were randomly assigned to either the intervention or control group, and were masked as to group assignment. Randomisation procedures: the investigator prepared 33 manila clasp envelopes; the contents included a sheet with the word Intervention printed on it...The envelopes were numbered sequentially from one through 33; this number served as the participant's identification number.... The investigator prepared another 33 manila envelopes; the content included a sheet with the words Standard care printed on it...The envelopes were numbered sequentially from 34 through 66; this number served as the participant's identification number. The investigator mixed all the envelopes into one batch and shuffled them 10 times. The investigator divided the shuffled envelopes into four piles, one pile for each home medical supply facility site (Sites A, B, C, and D).
According to this description, the investigator who prepared the envelopes would be aware of the allocation contained within each envelope based on viewing the outside of the envelope. It is not clear whether any other investigator/staff (e.g. those responsible for selecting the next envelope from the stack) was also aware of the sequence correlation (i.e. that lower numbers were assigned to intervention and higher numbers to control). Additionally, author reports that, due to withdrawal of sites from the study, "unused envelopes from Sites B, C, and D were taken to Site A." It is unclear who delivered those unused envelopes. There is insufficient information regarding allocation concealment to make a determination as to whether it was possible for enrolling investigators/staff to have knowledge of forthcoming allocation. Mean and SD for all key baseline characteristics (age, gender, BMI, AHI) were reported for all randomised participants. Authors reported that differences were non‐significant: "After comparing the frequencies (chi‐square tests) and descriptives (independent samples t‐tests) analyses by group, the groups were determined to be homogeneous. There were no statistically significant differences between the intervention and standard care groups as to general and sleep demographics." |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Authors report participants were "masked as to group assignment," but do not describe the methods by which this masking was carried out for a behavioral intervention. Authors report, "The initial number of sites was four (Sites A, B, C, and D). Two sites were lost to attrition (Sites C and D) due to the unavailability of a respiratory therapist to provide CPAP teaching. As these two sites withdrew from the study, recruitment slowed at Site B, and recruitment progressed at Site A, unused envelopes from Sites B, C, and D were taken to Site A. Additional envelopes were provided to sites as needed; all 66 envelopes were used." Though this is a deviation from planned randomisation protocol, it did not appear to result in a deviation from the intended intervention. Moreover, "[I]ntervention fidelity was maintained with the use of a protocol training manual and initial and booster training sessions to prepare the research assistants; monthly fidelity checks were conducted with no problems noted." (p. 102) From Methods, "If participants were noted to have values that appeared missing at random, their data was included in the analyses. Other missing data were evaluated on a case‐by‐case basis and excluded from analyses" From Results, "One intervention group participant was lost to attrition midway through the study. Missing data were determined to be missing at random and were included in the statistical analyses; where applicable, cases were excluded pairwise." These descriptions do not describe how missing data were 'included,' e.g. last observation carried forward, imputation, set to 0, excluded. However, there is no evidence that trialists used either 'as‐treated' or naive per‐protocol analysis. Rather, report suggests that all participants were analysed in the group to which they were randomised. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | 1 of 33 (intervention) and 0 of 33 (control) were lost to attrition. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. The distribution of Philips and ResMed devices were equal in each arm. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Low risk | Authors report, "Before data was collected, IRB approval and informed consent were obtained." Additionally, this is a dissertation and the author presents a comprehensive protocol with design and analytic plan. Thus, it is likely that analytic plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that two time points were planned; each planned outcome reported. Methods section indicates that one, commonly‐employed threshold adherence definition was planned; this outcome was reported in Results. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Smith 2006.
| Methods | Randomised parallel‐group trial | |
| Participants |
N = 19 with newly‐diagnosed OSA, non‐adherent with CPAP for 3 months Inclusion criteria: new OSA diagnosis, first CPAP prescription, received initial education on CPAP use and supplemental audiotaped/videotaped reinforcement at two and four weeks, non‐adherent with CPAP for 3 months Exclusion criteria (unclear if a priori): positive screen for drug or alcohol abuse, depression requiring hospitalisation Baseline characteristics: % female NR. Mean age 63 (± 8). Mean AHI NR. Mean ESS NR. Mean BMI NR. Country: USA |
|
| Interventions | Participants were randomised to control (n = 9) or intervention (n = 10) group. Intervention:two‐way telehealth sessions mediated by video link‐up through phone line. Research nurse emphasised nightly, bedtime routine for CPAP. After standardised protocols, nurse visually assessed participant, guided correct CPAP routine and determined whether the CPAP mask fits properly. Nurse described consequences of non‐adherence and managing barriers to CPAP use. Benefits of nightly CPAP use for general health were emphasised Control: two‐way telehealth sessions mediated by video link‐up through phone line. Protocols drawn up to mimic content delivered to intervention group. Instead of CPAP‐related information, participants given content on vitamin intake Study duration: 12 weeks |
|
| Outcomes |
|
|
| Notes | Non‐adherence in the study defined as less than four hours of CPAP use per night for fewer than nine of 14 consecutive nights' use TJL emailed for details of randomisation and outcome data 12 September 2008. Carol Smith responded 15 September 2008. For updated review, further email communication was required to verify that updated inclusion criteria were met, confirmation received from Carol Smith, 27 March 2019. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomised and done via computer software generated random assignment" |
| Allocation concealment (selection bias) | Low risk | "...allocation sequence and treatment group assignment concealed from investigators conducting the screening and ongoing assessments" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single‐blind; nursing interventionist staff aware of different content delivered by video link‐up Machine usage was measured via smart card by blinded sleep lab personnel. Information on participants' awareness of CPAP machine usage was insufficient for us to determine how this might have affected the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants finished follow‐up and contributed to data on adherence. Two satisfaction surveys were not submitted (one from each group) |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: "After the University of Kansas Medical Center's Institutional Review Board approval, the random assignment process began." No reference to random component or allocation concealment method. Regarding baseline characteristics, the authors provided no table. They reported, "Group 1 and group 2 were compared using two group t test statistics to assure there were no between‐group differences. Mean ages of the two groups did not differ. Patient ages ranged from 50 to 83, with a mean of 63 +/‐ 7.95. All patients' respiratory distress index (RDI) scores were all in the severe range with scores not differing significantly between groups 1 and 2 (t test = 0.737, P = 0.471). These results indicate there was no significant difference between group 1 and group 2 on age or severity of sleep apnoea. Thus, age or severity of sleep apnoea did not influence outcomes of adherence." Thus, authors report that differences in age and baseline OSA severity are consistent with chance. However, they do not report (and may not have evaluated) baseline differences in gender or BMI. There was no information on some potentially influential baseline characteristics. Given the date of the publication and the author affiliation with a VA hospital, as well as the small N, Review authors suspect this study was conducted on all male participants. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. Outcome based on all randomised participants based on denominators used for calculation of proportions adherent, "A higher percentage of group 1 than group 2 participants were adhering to CPAP after the telehealth interventions (X2=4.55, P = 0.033). Specifically, 90% (n = 9 of 10) of group 1 compared to 44% (n = 4 of 9) of group 2 participants were adherent after the telehealth sessions." |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Authors report, "...there were only 3 episodes of transmission problems, each easily corrected." This suggests that there were no missing outcome data. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | The adherence outcome measurement comes from participants' CPAP ventilator timer‐recorder. This is consistent with CPAP technology available at the time of the study. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups (verified via author correspondence). Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. Methods section indicates one outcome time point (for primary adherence outcome) was planned. Results section reports one outcome time point. Methods section indicates that one, commonly‐employed threshold adherence definition was planned; this outcome was reported in Results. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Smith 2009.
| Methods | Randomised parallel‐group trial | |
| Participants |
N = 97 patients with newly‐diagnosed OSA. Mean age: 63.4, male sex: 55%, Mean AHI: Intervention group: 52.3, Control group: 47.3 Inclusion criteria: new diagnosis of OSA, age ≥ 18, AHI ≥ 20 Exclusion criteria (unclear if a priori): positive screen for drug or alcohol abuse, depression requiring hospitalisation Baseline characteristics: 45% female. Mean age 63. Mean AHI 50.1. Mean ESS NR. Mean BMI NR. Country: USA |
|
| Interventions | Participants were randomised to control (n = 42) or CPAP Habit Intervention (Intervention, n = 55) group.All participants received usual education on OSA and demonstration of CPAP equipment Intervention: audiotaped music along with softly spoken directions on relaxation techniques and habit‐promoting instructions for using CPAP nightly. Participants received information packet, which included CPAP use reminder placard, handouts on benefits of CPAP adherence and health consequences of poor compliance, four‐week diary for recording experience with CPAP Control: audiotaped music along with spoken information about vitamins. Information packet was the same in format and length as the intervention group, but content was on vitamins Study duration: 6 months |
|
| Outcomes |
|
|
| Notes | * Indicates primary outcome analysed in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned using computerised random assignment programme |
| Allocation concealment (selection bias) | Low risk | Participants recruited by"'nurses who had no knowledge of group assignment" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single‐blind;"'...placebo intervention was used to mimic the daily activities in the experimental treatment..." CPAP usage was measured via smart cards by blinded personnel. Nurses administering experimental or placebo control interventions aware of different content of these interventions. Unclear whether participants were aware of machine usage monitoring. Personnel analysing data on compliance were blind to allocation of treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis but imbalanced N of dropouts: Intervention group: 11/55 (20%), Control group: 13/42 (31%) at six months. Unclear whether reasons for dropouts were balanced across groups |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods "Patients were randomly assigned to the intervention (n=55) or the placebo control group (n=42) using computerized random assignment program." No reference to random component or allocation concealment method. Key baseline characteristics (age, gender, BMI, AHI) were reported and differences were insignificant (P values reported), consistent with chance. Though not explicitly stated (and no Ns provided in baseline tables), these appeared to reflect all randomised participants. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Authors report, "Trained personnel used a software program to obtain nightly CPAP use of > or < 4 hours at 1, 2 and 6 months from these data cards. These personnel were blind to allocation of treatment." Additionally, "Patients were given pre‐paid, addressed envelopes for returning the completed diary pages to the research centre. Research nurses contacted patients by telephone monthly reminding them to use their respective intervention, write in their diary and return their diary pages by mail to the research centre. These nurses were blinded to the data and evaluations participants mailed in." Therefore research nurses responsible for delivering intervention were aware of intervention assignment. No deviations documented; none suspected based upon review. ITT. Authors report (in Table 13 caption), "No participants had dropped out of the study at one month post intervention. participants who dropped out from the study (numbers listed below) had either stopped using CPAP, had less than 4 hours of CPAP use, or were lost to contact. Per the intent‐to‐treat analysis all dropouts remained in each analysis. Dropouts lost to contact were counted as non‐adherent." |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Authors report three primary endpoints: 1, 3, 6 months. Outcome data were available for 97 of 97 at 1 month, 94 of 97 (96.9%) at 3 months and 73 of 97 (75.3%) at 6 months. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned; each planned outcome reported. Authors chose an uncommon threshold definition of CPAP adherence, "4 or more hours per night and at least 9 of each 14 nights of ventilator use." The published report also does not explicitly state how this standard was applied to the 'CPAP nightly use data' collected at 1‐, 3‐ and 6‐month endpoints. Review authors are not clear as to how proportion adherent was calculated at each endpoint. For example, was 1‐month adherence threshold set at 64.3% of nights for 30‐day or calendar month, or was it calculated based on a 28 day month to preserve multiples of 14 days? Each option would yield different adherence proportions. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Soares‐Pires 2013.
| Methods | Randomised, parallel‐group study. | |
| Participants |
N = 202 patients with OSAHS. Inclusion criteria: AHI ≥15 or ≥5 events per hour plus symptoms that included unintentional sleep episodes while awake, daytime sleepiness, unrefreshing sleep, fatigue, insomnia, gasping or choking, or loud snoring and/or apnoea described by the patient's bed partner. Exclusion criteria: lung disease, obesity hypoventilation syndrome, restrictive ventilatory syndromes, long‐term oxygen therapy, Cheyne–Stokes breathing pattern, central apnoea, cognitive disability. Baseline characteristics: 29.5% female. Median age 58.5. Median AHI 38. Median ESS 12. Median BMI 32. Country: Portugal |
|
| Interventions |
Education group: participants were assigned to a single group education session one month after beginning APAP therapy. Sessions were conducted by a pulmonologist, a psychologist, and a respiratory physiotherapist. Sessions included information regarding OSAHS, its symptoms and risks, APAP treatment, the importance of good adherence, and different machine interfaces. Patients were invited to share their experience on the use of APAP, and each patient's adherence reports were analysed and discussed. Patients' concerns, fears, and beliefs were also addressed. Standard Care: the sleep physician provided a brief explanation of the disease to patients of both groups, as well as informed patients of the need for APAP treatment, its benefits and function mode. None of the patients had previously received any form of PAP therapy. Approximately 3–5 days after the prescription, technicians from the PAP systems delivery companies performed a home visit to drop the APAP device. In this visit, an explanation on how to turn on and off the machine and on the placement of the interface was provided to all patients. Study duration: 6 months |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No reference to random component or allocation concealment method. |
| Allocation concealment (selection bias) | Unclear risk | No reference to random component or allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No evidence of blinding of participants, personnel or outcome assessors. Study only had objective outcomes, so lack of blinding is not likely affect outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were available/analysed for 146 of 202 (72.3%) randomised participants. |
| Selective reporting (reporting bias) | High risk | No protocol, abstract, clinical trials entry available for comparison. However likely that definition was chosen for its result. |
| Other bias | Unclear risk | Incomplete information available regarding baseline differences for variables of interest. No deviations from intended interventions reported or suspected. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within METHODS: "Patients were randomised into a study group and a control group. All patients in the study group were assigned to a single group education session, approximately 1 month after beginning APAP therapy (Fig. 1). Patients in the control group did not participate in the education session." No reference to random component or allocation concealment method. In the Methods (Study Design) section, and in the study design flow diagram (Figure 1), authors report that 202 participants were randomised to study arm, GI (n = 100) and control arm, G2 (n = 102). In the results section, authors report, "We evaluated 146 patients, 103 (70.5 %) males and 43 (29.5 %) females, with a median age of 58.5 years. Most patients were obese, hypertensive, and had severe OSAHS (Table 5). Baseline demographic and clinical characteristics did not differ in the study and control groups (Table 5)." This implies that Table 5 provides baseline characteristics and comparisons for only those who were analysed (n = 146) rather than for all randomised participants (n = 202). Therefore, cannot determine if baseline differences between intervention groups suggests a problem with randomisation. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. Probably mITT. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | Data were available/analysed for 146 of 202 (72.3%) randomised participants. Neither analyses to correct for bias nor sensitivity analyses were conducted. Missing outcome data occurred for undocumented reasons and, therefore, missingness could be related to the outcome. There are no significant differences between intervention arms in the proportions of missing outcome data. However, there are no reasons for missing outcome data other than 'dropped out,' so review authors cannot evaluate whether specific reasons provide evidence that missingness depends on true value or whether these reasons differ across intervention arms. Without this information, it is likely that those who are non‐adherent would be more likely to drop out and that, therefore, missingness depends on the true value of the outcome (adherence). Per Cochrane Handbook, 8.13.2.2, "Even if incomplete outcome data are balanced in numbers across groups, bias can be introduced if the reasons for missing outcomes differ." Since authors provide no information as to the reasons for dropouts, the potential for bias cannot be adequately assessed. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Unclear risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring. Authors reported using one of two device makes in the study (ResMed, Breas), but did not provide information as to the distributions of device make in each intervention arm. Outcome "assessor" is CPAP device: no knowledge of allocation possible. Unable to confirm with study author that distribution of CPAP device makes did not differ between intervention arms. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | High risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates one outcome time point (for primary adherence outcome) was planned. Results section reports one outcome time point. Authors report, "After 6 months of APAP therapy, usage data were downloaded by sleep technicians during a home visit. Adherence data, air leakage, air pressure delivered, and residual AHI were recorded. Adherence was analysed as a continuous and as a dichotomous variable. When analysed as a continuous variable, we recorded the percentage of days the APAP was used and the mean effective use per [*]effective day, defined as the cumulative time of effective use divided by the number of days APAP was actually used. When analysed as a dichotomous variable, we compared adherent versus non‐adherent. Patients were defined as being adherent if they used APAP at least 4 hours per night for at least 70 % of days." These outcomes were reported in results section. However, standard reporting for daily use is mean use per day over *all days in the study period. Mean use per effective day will result in upward bias in usage estimates. Since mean use per day was likely also calculated, the decision to report mean use per effective day suggests that the numerical result being assessed was selected on the basis of the results from multiple outcome measurements. Methods section indicates that one, commonly‐employed threshold adherence definition was planned; this outcome was reported in Results. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Sparrow 2010.
| Methods | Randomised parallel‐group trial | |
| Participants |
N = 250 patients undergoing initial set‐up of fixed‐pressure CPAP or BiPAP. Inclusion criteria: age 18 to 80 years, AHI > 10 Exclusion criteria: Not reported Baseline characteristics: 18% female. Median age 55. Median AHI 38.3. Median ESS 10.5. Median BMI 35.1. Country: USA |
|
| Interventions | Participants were randomised to control (n = 126) or interactive voice response system, TLC‐CPAP (TLC‐CPAP , n = 124) group. TLC‐CPAP: automated telephone‐linked communication system adapted for CPAP (TLC‐CPAP), designed around the concepts of motivational interviewing. Digitised human speech was used, and participants were communicating with it via touch tone keypad of their telephones. The TLC‐CPAP content included assessment of the participant's experience with CPAP, self‐reported machine use, feedback and counselling to enhance adherence and side effect management. Participants were required to make weekly calls to TLC‐CPAP during the first month and monthly thereafter. Printed reports were sent to the participant's physician. Participants were encouraged to contact physician directly if any excessive symptoms or side effects of treatment encountered Control: attention placebo control' group received general education on a variety of health topics via a telephone‐linked communication (TLC) system. Participants were required to make calls on the same schedule as the intervention group Study duration: 12 months |
|
| Outcomes |
|
|
| Notes | * Indicates primary outcome analysed in this Review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomisation stratified by sex, age and AHI using a randomised block design" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of participants attempted by developing an 'attention placebo control group'. However, given the nature of the intervention, participants may have been aware of group assignment. Participants in the intervention group self‐reported frequency and duration of CPAP usage. It is unclear whether participants in the control group were aware of CPAP usage monitoring "...all data were collected by research assistants blind to group assignment". Unclear whether the same applied to outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed by intention‐to‐treat. Multiple‐imputation procedure was implemented to account for missing data in the outcome of CPAP use due to loss to follow‐up. 20/124 in the intervention group and 15/126 in the control group lost to follow‐up at 12 months |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: " Participants were then randomised to one of two groups: one group used an educational control TLC system ('attention placebo control group') and the other group used the TLC‐CPAP system. Randomisation was stratified by sex, age and AHI using a randomised block design to ensure balance of these factors in the treatment arms." 250 participants were randomised: 124 to intervention, 126 to control. Key baseline characteristics (age, gender, BMI, AHI) were reported for all randomised participants and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Analyses were performed by intention‐to‐treat. No deviations documented; none suspected based upon review. Authors report, "Analyses were performed by intention to treat." Results report, "CPAP adherence data were available from either the 6‐ or 12‐month follow‐up visit in 93.6% of participants (figure 1), who were therefore included in the primary analysis." This suggests primary CPAP usage results were based on mITT analyses. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Unclear risk | Authors report, "CPAP adherence data were available from either the 6‐ or 12‐month follow‐up visit in 93.6% of participants (figure 1)." Participant flow shows that 110 of 124 (88%) randomised to intervention arm had CPAP adherence data at 6‐month endpoint; 112 of 126 (88%) in control arm. Despite the availability of outcome data for at least one endpoint (6‐ or 12‐month) in 93.6% of participants, each group had < 90% of outcome data at each endpoint. Moreover, authors did not provide information as to the reasons for missing data and there was a slight imbalance in missing data across arms at 12 months (83.8% in intervention arm, 88.1% in control arm). Per Cochrane Handbook, 8.13.2.2, "Even if incomplete outcome data are balanced in numbers across groups, bias can be introduced if the reasons for missing outcomes differ." Since authors provide no information as to the reasons for missing data, the potential for bias cannot be adequately assessed. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring. Unable to confirm with study author that distribution of CPAP device makes did not differ between intervention arms. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Low risk | Publication (2010) and ClinicalTrials.gov entry, NCT00232544 (first submitted 30 Sep 2005, last updated 10 Oct 2018) available and reviewed. Actual primary completion date is listed as 'March 2008 (final data collection date for primary outcome measure.' Original primary outcome (submitted 30 Sep 2005) was "Objective CPAP use and disease specific quality of life at 6 and 12 months" and current primary outcome (submitted 31 Dec 2013) is "Objective CPAP Use (Time Frame: 12 months) Mean nightly hours of CPAP use over 12 months." Published report (2010) includes both 6‐ and 12‐month endpoints. Though the trialists do not explicitly state that analysis plan was finalised before unblinded outcome data were available for analysis, the original primary outcomes were submitted well before primary completion date and are identical to outcomes reported in published report. Methods section indicates that multiple time points planned; each planned outcome reported. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Stepnowsky 2007.
| Methods | Randomised parallel‐group trial | |
| Participants |
N = 45 patients newly‐diagnosed with OSA. Inclusion criteria: AHI ≥ 15, no prior CPAP treatment, stable sleep environment Exclusion criteria: allergies/sensitivity to mask or mask material, previous use of any other PAP device (e.g. bi‐level PAP, auto‐adjusting PAP), current use of prescribed supplemental oxygen or significant comorbid medical conditions that could interfere with daily use of CPAP Baseline characteristics: 2% female. Mean age 59 (±14.3). Mean AHI 39. Mean ESS 12.6. Mean BMI 32.8. Country: USA |
|
| Interventions | Participants were randomised to usual care (UC, n = 24) or telemonitoring (TM, n = 21) group. TM: review of compliance and efficacy data. Monitored information garnered as objective compliance data and subjective reports of usage. Follow‐up tailored to how CPAP used by participants. Details on how many total hours the PAP unit was used each night at therapeutic pressure. Efficacy data consisted of the amount of mask leakage (L/s) and the AHI (total number of apneas and hypopnoeas per hour of sleep) UC: telephone call from staff one week after CPAP initiation and office follow‐up visit at one month. Participants encouraged to call clinic any time with problems or concerns Study duration: 2 months |
|
| Outcomes |
All outcomes reported at 2 months. |
|
| Notes | For original (Wozniak 2014) review: TJL emailed for randomisation 12/09/2008. Carl Stepnowsky responded 15/09/2008. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...we used the uniform random number generator in R to select all sequences of 4 randomly with equal probability so that the occurrence of 3 in a row being assigned to the same group would be extremely rare" |
| Allocation concealment (selection bias) | Low risk | "The randomisation scheme was concealed until the time at which the intervention was assigned. The randomisation scheme was generated by the project statistician and carried out by research staff immediately after the informed consent procedure and the completion of the baseline questionnaires" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants in both groups received a monitoring unit All participants likely to be aware that CPAP usage was measured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "There were five CPAP "rejectors," or patients who decided within the first day or two that they did not want to pursue CPAP as the primary treatment for their OSA. Our study did not have a "run‐in" period, which could have helped identify these patients prior to the intervention" |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: "The randomisation scheme was concealed until the time at which the intervention was assigned. The randomisation scheme was generated by the project statistician and carried out by research staff immediately after the informed consent procedure and the completion of the baseline questionnaires." No reference to random component or allocation concealment method. Baseline characteristics were presented (Table 13) for those participants who completed the study (n = 40), not for all randomised participants (n = 45). Key baseline characteristics (age, BMI, AHI) were reported for all participants who completed the study and differences were non‐significant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Unblinded trial. No deviations documented; none suspected based upon review. mITT. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | 40 of 45 (88.9%) randomised participants had outcome data at primary endpoint. Authors report all of these participants 'rejected' CPAP "within the first day or two," presumably after randomisation. Neither analyses to correct for bias nor sensitivity analyses were conducted. Missing outcome data occurred for documented reasons (rejected CPAP) that could be related to the outcome. 1 of 21 (4.8%) and 4 of 24 (16.7%) of control and intervention arms, respectively, rejected CPAP after randomisation. There were differences between intervention arms in the proportions of missing outcome data. Reason for missing outcome data (CPAP rejection within 1‐2 days after randomisation) is related to the outcome but did not differ between groups. It is likely that missingness in the outcome depended on its true value, which may have biased estimated effect. Favours experimental. CPAP rejectors would have zero hours of use, which would disproportionately reduce average CPAP usage/adherence in the experimental arm relative to the reduction in the control arm. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol available. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates one outcome time point (for primary adherence outcome) was planned. Results section reports one outcome time point. Methods section lists no threshold‐based adherence definition. Results table includes two threshold‐based adherence outcomes, one of which was also discussed in Results (text) section. These were likely selected after analysis of results, but were not presented as primary outcome of interest. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
Stepnowsky 2013.
| Methods | Randomised parallel‐group trial | |
| Participants |
N = 241 patients with a recent OSA diagnosis and prescription for CPAP therapy. Inclusion criteria: diagnosis of OSA (AHI ≥ 15), CPAP therapy prescription, and age ≥ 18 years. Exclusion criteria: residence in a geographical area outside of San Diego County, fatal comorbidity (life expectancy less than 6 months as indicated by physician); or significant documented substance/chemical abuse. Baseline characteristics: % female NR (may be all male veterans). Mean age 52.1 (± 13.3). Mean AHI 36.5. Mean ESS 10.6. Mean BMI 32.5. Country: USA |
|
| Interventions | Participants were randomised to telemonitoring (TM, n = 126) usual care (UC, n = 115) group. TM: main goals of MyCPAP intervention were to (a) allow both the patient and provider access to telemonitored adherence and efficacy data on a daily basis, (b) act on that data collaboratively to guide CPAP management and troubleshoot problems early and effectively, and (c) emphasise ways for the patient to express their preferences and needs UC: diagnostic sleep study, CPAP instruction and setup by trained healthcare provider, and follow‐up at predetermined times (1 week, 1 month) by CPAP clinic staff. Beyond these pre‐determined clinic contacts, patients were encouraged to call whenever they had a problem or concern. Study duration: 4 months |
|
| Outcomes |
Outcomes were reported at 2 and 4* months. |
|
| Notes | * Indicates primary outcome analysed in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No reference to random component provided. |
| Allocation concealment (selection bias) | Unclear risk | No reference to allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that outcome data are available for nearly all (> 95%) of participants. |
| Selective reporting (reporting bias) | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. |
| Other bias | Low risk | No baseline imbalances or reported/suspected deviations from intended intervention. Although gender was not reported, it was likely that this population was 100% male as it was conducted in a VA (Veterans Affairs) hospital in the USA. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: "The design was a randomised parallel group trial with blinded evaluation that compared an Internet intervention based on the wireless telemonitoring of CPAP data (i.e. Internet‐based positive airway pressure care, or MyCPAP) versus a usual care CPAP treatment protocol (i.e. Usual Care, or UC)." No reference to random component or allocation concealment method. Key baseline characteristics (age, BMI, AHI) were reported for all randomised participants and differences were insignificant (P values reported), consistent with chance. Gender was reported as an intended demographic characteristic to be collected, but gender distribution among participants was not reported. Since study conducted at Veteran's Hospital in US, suspect that all participants were male, but cannot confirm. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. Authors do not report if any outcome data were missing and, if so, how missingness was handled. Thus, analysis was either ITT or mITT. |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Authors report, "Two hundred forty‐one participants were enrolled over the project period (115 to Usual Care and 126 to the MyCPAP group).The total number of withdrawals during the course of the project was seven. These were due to CPAP intolerance or subsequent self‐withdrawal from the study." Baseline characteristics and 2‐ and 4‐month outcome results are reported for 126 (intervention) and 114 (control), respectively. Depending on whether the 7 participants who withdrew were included among the reported 241 enrolled, outcome data may be missing for 2.8% to 2.9% of participants. It is also possible that only one participant had missing data since results table lists group Ns as 126 (intervention) and 114 (control). Authors do not report on missingness. It is also unclear what, if any, data were missing at 2‐ and 4‐month endpoints and how the missingness was handled in reporting results. Regardless, it appears that outcome data are available for nearly all (> 95%) of participants. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned; each planned outcome reported. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Turino 2017.
| Methods | Prospective randomised controlled trial. | |
| Participants |
N = 100 newly diagnosed OSA patients Inclusion criteria: >18 years, newly diagnosed OSA requiring treatment with CPAP (AHI >15). Exclusion criteria: impaired lung function (overlap syndrome, obesity hypoventilation and restrictive disorders), severe heart failure, psychiatric disorders, periodic leg movements, pregnancy, other dyssomnias or parasomnias, history of previous CPAP treatment. Baseline characteristics: 23% female. Mean age 55 (NR). Mean AHI 52. Mean ESS NR. Mean BMI 35. Country: Spain |
|
| Interventions | Participants were randomised to standard management (SM, n = 48) or a telemonitoring programme (TM, n = 52) TM: each CPAP device equipped with mobile 2G technology capable of sending daily information on CPAP adherence, CPAP pressures, mask leak and residual respiratory events to the web database. Automatic alarms for the provider were generated in case of mask leak >30 L/minute for > 30% of the night or usage of < 4 hours/night on two consecutive nights. In case of alarm, the pulmonary specialist medical officer of the CPAP provider contacted the patient, providing case‐by‐case problem solving. SM: patients were fitted with a mask and given a CPAP device and a leaflet explaining how to use it. A short instruction session on CPAP device use was provided to patients and partners in the sleep unit by a trained nurse. This included a practical demonstration of how to put on the mask, and the correct management and cleaning of the tubes, masks and humidifier. Information on how to turn the CPAP device on and off was provided by the homecare provider at the time of machine delivery. All patients were visited after 1 month of treatment by the nurse at the sleep unit. |
|
| Outcomes |
|
|
| Notes | * Indicates primary outcome analysed in this Review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised to have CPAP therapy managed using standard care or a telemonitoring‐based strategy and followed up over 3 months." No reference to how randomisation was achieved. |
| Allocation concealment (selection bias) | Unclear risk | No reference to allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors do not report any similar outcome (dropouts, withdrawals, missing) in the published report. Authors report that all analyses would be performed on intent‐to‐treat and per‐protocol basis. However, they do not report per‐protocol results. Additionally, all results tables Ns correspond to randomised Ns. Thus, it is unclear if there were any missing outcome data and, if so, how that was handled in the derivation of primary mean CPAP usage/adherence. |
| Selective reporting (reporting bias) | Unclear risk | Dates provided in trial's NCT entry do not allow us to determine if analysis plan was finalised before unblinded outcome data were available for analysis. |
| Other bias | Low risk | No baseline imbalances or reported/suspected deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: "Patients were randomised to have CPAP therapy managed using standard care or a telemonitoring‐based strategy and followed up over 3 months." No reference to random component or allocation concealment method. Key baseline characteristics (age, gender, BMI, AHI) were reported for all randomised participants and differences were insignificant (P values reported), consistent with chance. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | Open‐label. No deviations documented; none suspected based upon review. ITT. Authors report, "All analyses were performed on both an intention‐to‐treat and a per‐protocol basis...," and "All results presented are for the intention‐to‐treat analysis...." |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | Low risk | Protocol (NCT02517346) includes 'abandons at 3 months: Number of patients lost at follow up at 3 months of CPAP therapy' as a secondary outcome. Authors do not report this or any similar outcome (dropouts, withdrawals, missing) in the published report. Authors report that all analyses would be performed on intent‐to‐treat and per‐protocol basis. However, they do not report per‐protocol results. Additionally, all results tables Ns correspond to randomised Ns. Thus, it is unclear if there were any missing outcome data and, if so, how that was handled in the derivation of primary mean CPAP usage/adherence. In Methods, authors report, "Given the high motivation of both professionals and patients to be involved, no dropouts were anticipated and thus a total of 100 patients were planned to be recruited." Thus, it is possible that there were no dropouts. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | Publication (2017), abstract (2017) and ClinicalTrials.gov entry, NCT02517346 available and reviewed. NCT information first submitted 30 July 2015, last updated 15 Apr 2016. Study start date reported as January 2015. Primary outcome (CPAP adherence, hours/night, at 3 months) submitted 04 Aug2015 and is the same as current primary outcome (i.e. no updates/changes were submitted to ClinicalTrials.gov by authors). Published report includes primary outcome specified in NCT entry and also includes 1 month compliance. Actual primary completion date was listed in NCT entry as: 'Sep 2015 (final data collection for primary outcome measure)'. Dates of recruitment not specified in NCT entry. Journal submission received by journal 7 June 2016, accepted after revision 20 Nov 2016. Dates provided do not allow us to determine if analysis plan was finalized before unblinded outcome data were available for analysis. Protocol and Methods section of published report indicate one outcome time point (for primary adherence outcome) was planned. Results section reports two outcome time points, including planned primary. No evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted. No threshold‐based adherence outcomes reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | Unclear risk | ‐ |
Wang 2012.
| Methods | Randomised parallel‐group study. | |
| Participants |
N = 152 participants with a new OSA diagnosis. Incusion criteria: new OSA diagnosis, AHI ≥ 10, above elementary school education, 'conscious mind and able to communicate clearly' Exclusion criteria: personal or family history of mental illness, drug or alcohol abuse, severe cognitive impairment, 'concurrent oncologic or psychiatric diseases' Baseline characteristics: 6.8% female. Mean age NR. Mean AHI 43.1. Mean ESS=14.1. Mean BMI NR. Authors did not report mean age for full sample or by intervention arm (reported only distribution Ns per (4) age groups for each arm). Also did not report average BMI for full sample or by intervention arm (reported only distrubution Ns per (4) BMI groups for each arm). Country: China |
|
| Interventions | Participants were randomised to one of four arms: PMR+EDU (n = 38), EDU (n = 38), PMR (n = 38), Control (n = 38). Education (EDU only): three nights of CPAP titration in the first week, 4‐hour group education session on OSA and CPAP in the first week, participants were given a brochure describing benefits of CPAP and CD containing a 20‐minute video demonstrating how to optimise CPAP treatment, 24‐hour consultation telephone line to the sleep nurses was available Progressive Muscle Relaxation Training (PMR only): one night of CPAP titration in the hospital, 12 × 40‐minute group Progressive Muscle Relaxation (PMR) practice sessions over 12 weeks, one per week. Self‐practice of PMR before each CPAP treatment. Brochure and CD with a guide for PMR practice at home. EDU + PMR: three nights of CPAP titration in the hospital. Combination of interventions as in Education and PMR group (see above) Control: one night of CPAP titration in the hospital in the first week Study duration: 12 weeks |
|
| Outcomes |
|
|
| Notes | Trialists included three intervention arms. One arm was Educational (EDU), one was Behavioral (PMR) and the third was Mixed (EDU+PRM). These were compared to control in respective meta‐analyses (i.e. Educational, Behavioral, Mixed). *Indicates primary outcome endpoint analysed in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomly assigned to a control group (C), an education group (E), a PMR group (P), and an education+PMR group (E+P) by block randomisation, resulting in 38 patients each group." No reference to how randomisation was achieved. |
| Allocation concealment (selection bias) | Unclear risk | No reference to allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants, personnel or outcome assessors. Study had subjective outcomes, so knowing group assignment could influence these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 47.4% (Control) to 78.9% (E+P) of participants, corresponding to an overall availability of 63.8% across groups. |
| Selective reporting (reporting bias) | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. |
| Other bias | Low risk | No baseline imbalances or reported/suspected deviations from intended intervention. |
| Bias arising from the randomisation process (ROB2, primary outcome) | Unclear risk | Only information provided as to the randomisation procedures used was within Methods: "The patients were randomly assigned to a control group (C), an education group (E), a PMR group (P), and an education+PMR group (E+P) by block randomisation, resulting in 38 patients each group." No reference to random component or allocation concealment method. Key baseline characteristics (age, gender, AHI) were reported for all randomised participants and differences were insignificant (P values reported), consistent with chance. BMI was not presented and compared across intervention arms as a continuous variable; rather, distribution across derived BMI categories were compared across arms. In this comparison, there was no statistical difference in distribution. |
| Bias due to deviations from intended interventions (ROB2, primary outcome) | Low risk | No deviations documented; none suspected based upon review. Authors report, "The patients' CPAP adherence rates and dropout rates were analysed on an intention‐to‐treat basis. The nonadherent patients were those who either dropped out of the study or those who stayed in the study but only used CPAP for a fraction of the required time. All dropouts remained in the analysis." |
| Bias due to missing outcome data (ROB2, primary outcome) All outcomes | High risk | At 12 weeks (final endpoint assessed), outcome data were available for 47.4% (Control) to 78.9% (E+P) of participants, corresponding to an overall availability of 63.8% across groups. Neither analyses to correct for bias nor sensitivity analyses were conducted. Missing outcome data occurred due to study dropout, which could be related to the outcome. Therefore, it is possible that missingness in the outcome was influenced by its true value. Missing data (due to dropout) proportions differed by intervention arm at each endpoint. Though missing outcome data did not differ across groups, the only reason cited for missing outcome data was study dropout, which is likely to be related to the true value of the outcome. Thus, missingness in the outcome is likely dependent on its true value. |
| Bias in measurement of the outcome (ROB2, primary outcome) All outcomes | Low risk | Outcome measured using objective CPAP usage data. Each intervention group outcome data ascertained via automated CPAP device monitoring; devices identical or sufficiently similar (i.e. similar distributions of CPAP device make) across groups. Outcome "assessor" is CPAP device: no knowledge of allocation possible. |
| Bias in selection of the reported result (ROB2, primary outcome) All outcomes | Unclear risk | No protocol, abstract, clinical trials entry available for comparison. Results presented were in accordance with the plan specified in the Methods section of the publication. No information as to whether analysis plan was finalised before unblinded outcome data were available for analysis. Methods section indicates that multiple time points planned; each planned outcome reported. Through a threshold definition of "4 or more hours per night and at least 9 of each 14 nights of ventilator use" is uncommon relative to the more common definition (> 4 hours/night on at least 70% of nights), there is no evidence that multiple analyses (e.g. variable adherence 'thresholds') were conducted and only this one was reported. |
| Overall risk of bias (ROB2, primary outcome) Machine usage | High risk | ‐ |
AHI: Apnoea Hypopnoea Index; APAP: Automatic positive airway pressure; BAI: Beck Anxiety Inventory; BiPAP: Bi‐level positive airway pressure; BMI: Body Mass Index; BP: Blood pressure; CHF: Congestive Heart Failure; COPD: Chronic Obstructive Pulmonary Disease; CBI: cognitive behavioural therapy; CPAP: Continuous positive airway pressure; CSA: Central Sleep Apnoea; CVD: cardiovascular disease;DASS: Depression Anxiety Stress Scales; ESS: Epworth Sleepiness Scale; ESRD: End‐Stage Renal Disease; FOSQ: Functional Outcomes of Sleep Questionnaire; HADS: Hospital Anxiety and Depression Scale; HSAT: Home sleep Apnoea testing; ICSD: International Classification of Sleep Disorders; IQR: Interquartile range; LCOF: last observation carried forward; LTOT: long‐term oxygen therapy; mITT: Modified intention‐to‐treat; MSLT: Multiple sleep latency test; ODI: Oxygen saturation index; OSA: Obstructive sleep apnoea; OSAHS: Obstructive sleep apnoea‐hypopnoea syndrome; PAP: Positive airway pressure; PBS: Peer buddy system; PSG: Polysomnography; PSQI: Pittsburgh Sleep Quality Index; QoL: Quality of life; RCT: randomised controlled trial; RDI: Respiratory Disturbance Index; SAQLI: Sleep apnoea Quality of Life Index; SEMSA: Self‐efficacy in sleep apnoea; SF‐36: Short‐Form health survey, 36 items; SOC: Standard of care; TIA: Transient Ischaemic attack; TLC‐CPAP: Telephone‐linked communications‐continuous positive airway pressure; VA: Veterans Affairs.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aloia 2005 | Inconsistent CPAP make or pressure delivery mode. |
| Andreu 2012 | Suspected OSA (not all participants received dx evaluation). |
| Andrews 2010 | Wrong intervention. |
| Antic 2009 | Wrong intervention. |
| Arrua 2016 | Suspected OSA (not all participants received dx evaluation). |
| Bague 2015 | Inclusion/exclusion criteria not verified/no author response. |
| Bague‐Cruz 2014 | Inclusion/exclusion criteria not verified/no author response. |
| Baltzan 2014 | Wrong intervention. |
| Barbe 2013 | Wrong intervention. |
| Barbe 2014 | Wrong intervention. |
| Berry 2008 | Wrong intervention. |
| Billings 2010 | Wrong intervention. |
| Bittencourt 2015 | Wrong intervention. |
| Cartwright 2017 | Inclusion/exclusion criteria not verified/no response from author. |
| Chai‐Coetzer 2011 | Wrong intervention. |
| Chai‐Coetzer 2012 | Wrong intervention. |
| Chai‐Coetzer 2013 | Wrong intervention. |
| Cotton 2012 | Inclusion/exclusion criteria not verified/no response from author. |
| Damjanovic 2005 | No randomisation or randomisation not verifiable. |
| Damjanovic 2009 | No randomisation or randomisation not verifiable. |
| Dawson 2015 | Inclusion/exclusion criteria not verified/no response from author. |
| Deng 2013 | Inconsistent CPAP make or pressure delivery mode. |
| Di Elia 2008 | Wrong study design. |
| Engleman 1993 | Intervention could not be classified. |
| Epstein 2000 | Inclusion/exclusion criteria not verified/no response from author |
| Escourrou 2012 | Inclusion/exclusion criteria not verified/no response from author. |
| Fields 2016 | OSA diagnosis not verified. |
| Fletcher 1991 | Wrong study design. |
| Fresnelli 2016 | No randomisation or randomisation not verifiable. |
| Gupta 2011 | Wrong intervention. |
| Ha 2015 | Controls received intervention not provided to intervention arm. |
| Harris 2014 | Inclusion/exclusion criteria not verified/no response from author. |
| Hayes 2009 | Wrong intervention. |
| Hood 2013 | OSA diagnosis not verified. |
| Hostler 2014 | No randomisation or randomisation not verifiable. |
| Hostler 2017 | No randomisation or randomisation not verifiable. |
| Hwang 2014 | No randomisation or randomisation not verifiable. |
| Igelstrom 2018 | Wrong outcomes. |
| Ip‐Buting 2017 | OSA diagnosis not verified. |
| Isetta 2014a | Inclusion/exclusion criteria not verified/no response from author. |
| Isetta 2014b | Wrong outcomes. |
| Isetta 2015 | Inclusion/exclusion criteria not verified/no response from author. |
| Jones 2016 | Inclusion/exclusion criteria not verified/no response from author. |
| Jurado‐Gamez 2015 | No randomisation, randomisation not verifiable. |
| Kataria 2017 | Inclusion/exclusion criteria not verified/no response from author |
| Klein 2010 | No randomisation, randomisation not verifiable. |
| Kreutzer 2003 | Wrong study design. |
| Kuna 2011 | OSA diagnosis not verified. |
| Kuna 2015 | Inconsistent CPAP make or pressure delivery mode. |
| Kushida 2011 | Wrong intervention. |
| Lang 2018 | Wrong outcomes. |
| Lettieri 2009 | Wrong intervention. |
| Lopez‐Martin 2005 | Wrong comparator. |
| Luyster 2018 | Inclusion/exclusion criteria not verified/no response from author. |
| Marques 2017 | Inclusion/exclusion criteria not verified/no response from author. |
| Marshall 2003 | No randomisation, randomisation not verifiable. |
| Moore 2012 | Inclusion/exclusion criteria not verified/no response from author. |
| Nadeem 2013 | Inconsistent CPAP make or pressure delivery mode. |
| NCT00310310 | Wrong outcomes. |
| NCT00873977 | Wrong intervention. |
| NCT00939601 | Wrong study design. |
| NCT01013207 | Wrong intervention. |
| NCT01102920 | Wrong outcomes. |
| NCT01535586 | Wrong study design. |
| NCT01538069 | Wrong outcomes. |
| NCT01569022 | Wrong study design. |
| NCT01924507 | Wrong outcomes. |
| NCT01960465 | No randomisation, randomisation not verifiable |
| NCT02085720 | Wrong study design. |
| NCT02278094 | Wrong intervention. |
| NCT02331992 | Wrong study design. |
| NCT02339597 | Wrong intervention. |
| NCT02375321 | Inclusion/exclusion criteria not verified/no response from author. |
| NCT02459548 | Wrong intervention. |
| NCT02509247 | No randomisation or randomisation not verifiable. |
| NCT02553694 | Inclusion/exclusion criteria not verified/no response from author. |
| NCT02553902 | Wrong outcomes. |
| NCT02755831 | Wrong outcomes. |
| NCT02779894 | Wrong outcomes. |
| NCT02953028 | Wrong intervention. |
| NCT03007745 | Wrong intervention. |
| NCT03202602 | Wrong study design. |
| NCT03472612 | Wrong study design. |
| Palmer 2004 | OSA diagnosis not verified. |
| Pepin 1996 | Wrong intervention. |
| Pepin 1999 | Intervention could not be classified. |
| Pepin 2018 | Wrong study design. |
| Rodgers 2015 | Inclusion/exclusion criteria not verified/no response from author. |
| Ruhle 1999 | Wrong study design. |
| Sanchez‐de‐la‐Torre 2015 | Intervention could not be classified. |
| Sanchez‐Quiroga 2018 | Wrong intervention. |
| Schiefelbein 2005 | Inclusion/exclusion criteria not verified/no response from author. |
| Signes‐Costa 2005 | Wrong intervention. |
| Singhal 2016 | Inclusion/exclusion criteria not verified/no response from author. |
| Tarasiuk 2012 | Wrong outcomes. |
| Tarraubella 2017 | Suspected OSA (not all participants received dx evaluation). |
| Tatousek 2015 | Inclusion/exclusion criteria not verified/no response from author. |
| Taylor 2006 | Inclusion/exclusion criteria not verified/no response from author. |
| Wenzel 2008 | Wrong intervention. |
| Wiese 2005 | OSA diagnosis not verified. |
| Williams 2014 | Wrong outcomes. |
CPAP: continuous positive airway pressure; OSA: obstructive sleep apnoea.
Characteristics of studies awaiting assessment [ordered by study ID]
Alessi 2018.
| Methods | Randomised parallel‐group trial |
| Participants | n = 125 Veterans aged ≥ 50 years (mean age 63, 96% male, 42% non‐Hispanic white) with OSA and chronic insomnia |
| Interventions | Behavioural Insomnia Therapy + PAP adherence program vs. general sleep education program |
| Outcomes | Sleep (sleep onset latency (SOL‐d, minutes to fall asleep), Wake after sleep onset (WASO‐d, and Sleep Efficiency (SE‐d, Time asleep/time in bed) by sleep diary; Pittsburgh Sleep Quality Index (PSQI) Sleep Efficiency by actigraphy (SE‐a)) and objective PAP adherence (mean hours use/night (PAPhrs) and number of nights used ≥ 4hrs (PAPnts)) |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm inclusion criteria at this time |
Aloia 2013a.
| Methods | Randomised parallel‐group trial |
| Participants | n = 11 Newly diagnosed adults with moderate to severe OSA |
| Interventions | Education + a personalised video of apneic events vs. education + a standard video |
| Outcomes | Self‐reported risk perception, objective PAP adherence |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
Becker 2012.
| Methods | Randomised parallel‐group trial |
| Participants | n = 160 Patients undergoing ambulatory PSG who were diagnosed with OSA |
| Interventions | Supportive respiratory therapist based follow up vs. standard follow‐up |
| Outcomes | Cumulative PAP compliance at 1, 2 and 3 months, cost‐analysis |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
ChiCTR‐ONC‐17013132.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No available abstract or trial details in archive search. Reason for "Awaiting Classification" Status: no published report/data |
Chin 2018.
| Methods | Randomised parallel‐group trial |
| Participants | n = 491 Inclusion criteria for participation were: 1) diagnosis of OSA by an overnight sleep study, 2) started CPAP more than three months previously; and 3) visited the sleep centre or clinic every one or two months. Results: men n = 424, mean age = 60+/‐13 years old |
| Interventions | 1) Telemonitoring + clinic visit every 3 months vs. 2) Clinic visits every 3 months vs. 3) Clinic visits ever 1 month |
| Outcomes | Data on adherence to CPAP were analysed for 6 months. |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time, Results data for this study not currently available |
Daaz‐Cambrilles 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No available abstract or trial details in archive search. Reason for "Awaiting Classification" Status: no published report/data |
Deng 2017.
| Methods | Randomised parallel‐group trial |
| Participants | n = 48, severe adult OSAHS patients |
| Interventions | Sleep apnoea monitoring management platform vs. traditional CPAP card reader mode |
| Outcomes | Compliance, mean blood oxygen saturation, titration pressure, Epworth sleepiness scale after 1, 3, 6 and 12 months of treatment |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time |
Duncan 2018.
| Methods | Randomised parallel‐group trial |
| Participants | n = ?. Patients attending a Sleep Clinic with a diagnosis of OSA |
| Interventions | Patient‐Centered Sleep Study Report (Myhill 201SR) vs. usual care |
| Outcomes | Perceived Efficacy in Patient‐Physician Interactions scale (PEPPI‐5), Apnoea Knowledge Test (AKT) and Self Care Management tool (SCM). Objective compliance to CPAP therapy will be collected at three months following the sleep study. |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time, Results data for this study not currently available |
Georgoulis 2018.
| Methods | Randomised parallel‐group trial |
| Participants | n = 108 Adult overweight patients (79% men, mean age: 47+/‐10 years, mean body mass index (BMI): 35.9 ± 6.2 kg/m2] with polysomnography‐diagnosed moderate‐to‐severe OSA (mean apnoea‐hypopnoea index (AHI): 59.6+/‐31.6 events/hour) |
| Interventions | Weight‐loss Mediterranean lifestyle intervention (MLI) combined with CPAP vs. CPAP alone |
| Outcomes | Patients were evaluated pre‐ and post‐intervention with regard to polysomnographic data, OSA symptomatology, anthropometric indices and lifestyle habits. |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time, unable to confirm if nightly machine usage was a study outcome |
Kataria 2018.
| Methods | Randomised parallel‐group trial |
| Participants | n = 19 Veterans with chronic stage TBI and newly diagnosed OSA |
| Interventions | Intensive education at the initial visit and nightly text message reminders to use PAP vs. standard‐of‐care |
| Outcomes | Mean percentage overall PAP compliance was averaged over the first seven days and at one month. The Epworth Sleepiness Scale (ESS) and cognitive tests were administered at baseline and 1 month. |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time |
Liaw 2018.
| Methods | Randomised parallel‐group trial |
| Participants | n = 20 Patients diagnosed with OSA and prescribed CPAP therapy. |
| Interventions | Long‐term follow‐up with GP and CPAP therapist vs. standard follow‐up with sleep physician |
| Outcomes | CPAP compliance, trial feasibility |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time |
Marques 2018.
| Methods | Randomised parallel‐group trial |
| Participants | n = 73 PAP‐naive adult patients with moderate to severe OSA; mean age was 57.2 years, mean AHI was 38,4 events/hour, and 59% of patients were male. |
| Interventions | Standard care consisting of first week, first, third and sixth month consultations vs daily telemonitoring information (i.e. adherence, air leak, residual AHI) plus standard care |
| Outcomes | Machine usage (hours/night), % participants deemed adherent (at least 5 hours per night for at least 90% of the days monitored), correlation between adherence and cardio‐metabolic clinical parameters. |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time |
Murase 2018.
| Methods | Randomised parallel‐group trial |
| Participants | n > 500; OSA patients |
| Interventions | Telemedicine vs. standard care |
| Outcomes | Objective CPAP adherence |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time, Results data for this study not currently available |
Naik 2015.
| Methods | Randomised parallel‐group trial |
| Participants | n = 30 Veterans naive to CPAP with confirmed OSA |
| Interventions | Wireless care with frequent modem monitoring vs. usual care |
| Outcomes | PAP adherence (more than 4 hours per night 70% of the time) |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT01259440.
| Methods | Randomised parallel‐group trial |
| Participants | n = 23 Adults with a confirmed diagnosis of moderate‐severe OSA, newly prescribed CPAP therapy with chronic symptoms noted on screening check list |
| Interventions | Supportive video teleconferencing vs. usual care |
| Outcomes | Nightly CPAP adherence over two months |
| Notes | Reason for ""Awaiting Classification" Status: no published report/data |
NCT01642160.
| Methods | Randomised parallel‐group trial |
| Participants | n = 50 Adults newly diagnosed with OSA who have been recommended to CPAP therapy |
| Interventions | Internet based education with weekly electronic follow up vs. usual care |
| Outcomes | Change in number of nursing interventions at 1 month |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT01715194.
| Methods | Randomised parallel‐group trial |
| Participants | n = 240 Symptomaticadults with OSA and an AHI and ODI of > 5/hour |
| Interventions | Telemonitoring with nursing contact as needed vs. usual care |
| Outcomes | Proportion of nights with CPAP use (>1hour/night), average nightly CPAP use |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT01785303.
| Methods | Randomised parallel‐group trial |
| Participants | n = 121 Adults newly diagnosed with OSA with co‐morbid insomnia |
| Interventions | Multidisciplinary approach to OSA (CBT for Insomnia + sleep diaries) vs. usual care |
| Outcomes | CPAP adherence at 3 months, Pittsburgh Sleep Quality Index , improvement in PSG sleep efficiency, Insomnia Severity Index, Functional Outcomes of Sleep Questionnaire, actigraphy |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT01796769.
| Methods | Randomised parallel‐group trial |
| Participants | n = 936 Sleep apnoea patients with low cardiovascular risk, newly treated with CPAP |
| Interventions | Telemonitoring web platform vs. usual care |
| Outcomes | Change in CPAP compliance at 6 months, Eppworth Sleepiness Scale, Pichot Scale, quality of life (SF‐12 questionnaire), cost‐analysis |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT01848509.
| Methods | Randomised parallel‐group trial |
| Participants | n = 200 Patients with OSA |
| Interventions | Telemonitoring system with alerts vs. usual care |
| Outcomes | CPAP use (hours/night), cost estimation, quality of life (SF 36, Functional Outcomes of Sleep Questionnaire) |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT01916655.
| Methods | Randomised parallel‐group trial |
| Participants | n = 360 Patients newly diagnosed with OSA |
| Interventions | Self‐management support protocol vs. mobile self‐management support protocol vs. usual care |
| Outcomes | Objective PAP adherence, self reported OSA symptoms, basic cost analysis |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT02056002.
| Methods | Randomised parallel‐group trial |
| Participants | n = 362 |
| Interventions | Peer buddy visits, both scheduled and as needed contacts vs. usual care |
| Outcomes | Patient satisfaction, CPAP adherence (hours/night, proportion of nights over 4 hours), self‐efficacy (SEMSA), Functional Outcomes of Sleep Questionnaire, Epworth Sleepiness Scale |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT02159885.
| Methods | Randomised parallel‐group trial |
| Participants | n = 53 Veteran adults with AHI > 5 as determined by home sleep study initiating APAP therapy |
| Interventions | Telemonitoring of APAP machine with phone follow up as needed vs. usual care |
| Outcomes | Daily APAP use (hours/night), change in Functional Outcomes of Sleep Questionnaire, cost analysis, Epworth Sleepiness Scale, depression (CES‐D), overall health (SF‐12), number of days CPAP adherent (> 4 hours) |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT02641496.
| Methods | Randomised parallel‐group trial |
| Participants | n = 144 Adults diagnosed with OSA by a sleep medicine physician with co‐morbid post traumatic stress disorder (PTSD) |
| Interventions | Cognitive behavioural therapy for OSA vs. sleep education |
| Outcomes | Time in hours of "mask‐on" CPAP use per night, Functional Outcomes of Sleep Questionnaire, PTSD Checklist |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT02657304.
| Methods | Randomised parallel‐group trial |
| Participants | n = 120 Adults with AHI > 30/hour or between 15‐30/hour with a Sleep Fragmentation Index of more than 10/hour |
| Interventions | Early education and monthly telephone calls vs. usual care |
| Outcomes | Percentage of participant with mean duration of CPAP >5 hours/night |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT03116958.
| Methods | Randomised parallel‐group trial |
| Participants | n = 60 Adults diagnosed with OSA and prescribed CPAP treatment |
| Interventions | Telemedicine via personalised smart phone application vs. usual care |
| Outcomes | Objective CPAP compliance (hours/night), number of nights adherence (> 4 hours/night), patient satisfaction, quality of life (EuroQOL), Epworth Sleepiness Scale, cost effectiveness |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT03243487.
| Methods | Randomised parallel‐group trial |
| Participants | n = 250 Adults with diagnostic AHI ≥15 as determined by PSG or home sleep test |
| Interventions | Structured patient adherence management system (phone calls based on recorded adherence) vs. usual care |
| Outcomes | Proportion of nights adherent (> 4 hours/night), nightly CPAP use (hours/night), participant satisfaction, cost analysis |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT03345524.
| Methods | Randomised parallel‐group trial |
| Participants | n = 145 Adults with suspected OSA who were referred for sleep testing |
| Interventions | Peer buddy system vs. usual care |
| Outcomes | Proportion of patients who followed through with sleep testing, nightly CPAP use |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT03446560.
| Methods | Randomised parallel‐group trial |
| Participants | n = 560 Adults with a verified OSA diagnosis indicated for CPAP treatment |
| Interventions | Telemedicine follow up customised according to objective CPAP use vs. usual care |
| Outcomes | Nightly CPAP use (hours/night), Epworth Sleepiness Scale, change in AHI, proportion of patients refusing CPAP, health care utilisation, patient satisfaction, Hospital Anxiety and Depression Scale, Insomnia Severity Index |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT03536572.
| Methods | Randomised parallel‐group trial |
| Participants | n = 250 Adults with confirmed diagnosis of OSA with chronic symptoms, newly prescribed for CPAP therapy |
| Interventions | Individualised pressure adjustment vs. usual care |
| Outcomes | CPAP adherence (hours/night) |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
NCT03792880.
| Methods | Randomised parallel‐group trial |
| Participants | n = 60 Inclusion criteria: 1) Patients with a recent diagnostic of OSAHS with AHI ≥ 30 and indication of CPAP, 2) age,≥ 18 years, 3) Few symptoms,without hyper somnolence (Epworth sleepiness scale ≤ 10) 4) Absence of clinic suspect or confirmation of other sleep pathology. 5)With interest in the use of new technologies |
| Interventions | telemedicine vs. standard care |
| Outcomes | % patients with a CPAP compliance ≥4 hours per day, CPAP compliance, dropouts, side effects, ESS, change in snoring, frequency of refreshing sleep, air leaks and residual AHI, EuroQoL, circadian rhythm parameters, cost‐effectiveness |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time, Results data for this study not currently available |
NCT03835702.
| Methods | Randomised parallel‐group trial |
| Participants | n = 166. Inclusion criteria: 1) >18 years old, 2) moderate to severe OSA (apnoea hypopnoea index (AHI) ≥15 events/hour) on polysomnogram (PSG), 3) Followed by non‐sleep providers for OSA (mainly primary care providers), 4) New PAP set up < 1 month. 5) Sub‐optimal PAP adherence by objective PAP adherence data (<70 % usage and <4 hours of average PAP usage) |
| Interventions | Sleep Apnea Management (SAM) Clinic vs. Usual Care with the non‐sleep provider |
| Outcomes | Hours of PAP usage (>4 hours), % days of PAP use in, ESS, PHQ‐9, PROMIS Global Health, PROMIS Fatigue, PROMIS Sleep Related Impairment, PAP Barrier questionnaire |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time, Results data for this study not currently available |
Nilius 2012.
| Methods | Randomised parallel‐group trial |
| Participants | n = 84 Adults with newly diagnosed OSA |
| Interventions | Intensive inpatient education + weekly phone calls and follow up vs. Intensive inpatient education + standard follow‐up |
| Outcomes | Average daily CPAP use (hours/night), Epworth Sleepiness Scale, CPAP rejection |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
Ordonez‐Dios 2013.
| Methods | Randomised parallel‐group trial |
| Participants | n = 166 Adults newly diagnosed with OSA who were eligible for CPAP treatment |
| Interventions | CPAP installation at CPAP‐school vs. CPAP installation in home |
| Outcomes | Objective CPAP adherence (hours/night), Epworth Sleepiness Scale, AHI |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
Pak 2016.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No available abstract or trial details in archive search. Reason for "Awaiting Classification" Status: no published report/data |
Park 2014.
| Methods | Randomised parallel‐group trial |
| Participants | n=50 CPAP naive adults with OSA |
| Interventions | Web based education portal vs. usual care |
| Outcomes | CPAP adherence (hours/night), proportion of days with > 4 hours CPAP, participant satisfaction |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
Peach 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No available abstract or trial details in archive search. Reason for "Awaiting Classification" Status: no published report/data |
Royant‐Parola 2013.
| Methods | Randomised parallel‐group trial |
| Participants | n = 200 |
| Interventions | Insufficient information provided in initial abstract |
| Outcomes | Insufficient information provided in initial abstract |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
Schoch 2017.
| Methods | Randomised parallel‐group trial |
| Participants | n = 240 Symptomatic adults with OSA consenting to long term CPAP therapy |
| Interventions | Telemedicine monitoring with as needed nursing follow up vs. usual care |
| Outcomes | Proportion of nights with CPAP use (> 1 hour/night), average nightly CPAP use (hours/night), Quebec Sleep Quality index, Epworth Sleepiness Scale |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
Shaikh 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No available abstract or trial details in archive search. Reason for "Awaiting Classification" Status: no published report/data |
Stepnowsky 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No available abstract or trial details in archive search. Reason for "Awaiting Classification" Status: no published report/data |
Stepnowsky 2014a.
| Methods | Randomised parallel‐group trial |
| Participants | n = 280 Patients diagnosed with OSA and prescribed CPAP therapy |
| Interventions | Individualised self‐management (ISM) vs. telemonitoring care (TC) vs. combined (ISM +TC) vs. usual care |
| Outcomes | Average CPAP use (hours/night) |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
Stepnowsky 2014b.
| Methods | Randomised parallel‐group trial |
| Participants | Insufficient information in abstract |
| Interventions | Video teleconferencing follow up vs. usual care |
| Outcomes | Objective CPAP adherence (hours/night), Epworth Sleepiness Scale, self reported OSA symptoms |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
Suarez 2017.
| Methods | Randomised parallel‐group trial |
| Participants | Insufficient information in abstract |
| Interventions | Telemonitoring vs. usual care |
| Outcomes | CPAP compliance (unspecified), Epworth Sleepiness Scale, ambulatory blood pressure, actigraphy |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
Tolson 2016.
| Methods | Randomised parallel‐group trial |
| Participants | n = 23 Adults recruited from a clinical sleep laboratory who were commencing CPAP therapy |
| Interventions | Group CBT targeting self efficacy vs. usual care |
| Outcomes | CPAP usage (unspecified), Self‐efficacy Measure for Sleep Apnea |
| Notes | Reason for "Awaiting Classification" Status: no published report/data |
Van Der Kleij 2018.
| Methods | Randomised parallel‐group trial |
| Participants | n = 115; patients with newly diagnosed OSA; Mean age (intervention vs control resp. 54;54 years), apnoea‐hypopnoea‐index (AHI, 47;48/hour), BMI (33;31 kg/m2), sex (man 75;74%), ESS (12;14) and FSS (37;37) |
| Interventions | Remote monitoring and adjustment using AirViewTM versus placebo |
| Outcomes | Compliance after 10 weeks and 1 year (CPAP use in mean hours/night), withdrawals |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time |
Zaldivar 2009.
| Methods | Randomised parallel‐group trial |
| Participants | n = 128 Adults with suspected OSA who were referred for sleep study |
| Interventions | Weekly phone calls vs. usual care |
| Outcomes | CPAP compliance (unspecified) |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time |
AHI: apnoea hypopnoea index; BMI: body mass index; BP: blood pressure; CBT: cognitive behavioural therapy; CPAP: continuous positive airway pressure; ODI: oxygen desaturation index; OSA: obstructive sleep apnoea; PAP: positive airway pressure; PSG: polysomnography ;TBI: traumatic brain injury.
Characteristics of ongoing studies [ordered by study ID]
Abreu 2013.
| Trial name or title | Evaluation of wireless telemonitoring of CPAP therapy in obstructive sleep apnoea ‐ TELEPAP study |
| Methods | Randomised, controlled clinical trial |
| Participants | 51 patients (42 males; mean age: 54 years old; mean apnoea/hypopnoea index (AIH): 36.8/h) newly diagnosed with OSA |
| Interventions | Standard clinical care (SC) vs active weekly phone call care (Myhill 201) (n = 18) vs. telemonitored clinical care (TC) (n = 12) with the use of Restraxx™ |
| Outcomes | Objective CPAP usage (hour per night), residual AHI |
| Starting date | 2013 |
| Contact information | |
| Notes | Author correspondence (21 Feb 2019): manuscript currently under review for publication. |
Bakker 2017.
| Trial name or title | A randomised trial demonstrating the feasibility of a group‐based peer‐support intervention for maximizing CPAP adherence |
| Methods | Randomised, controlled trial |
| Participants | N = 40; aged >21 years, newly diagnosed with OSA; 70% male, median age = 52 years [IQR=39‐62] |
| Interventions | Group‐based behavioral intervention delivered by trained 'patient advocates' (successful CPAP users) x two 90‐minute sessions vs standard care |
| Outcomes | % of participants using machine < 4 hours per night over 6 weeks, % agreement that attendance of peer support group was helpful |
| Starting date | 2015 |
| Contact information | |
| Notes | Author correspondence (25 Jan 2019): data currently available through NCT entry NCT02538419, results have yet to be published as a full manuscript. |
Castronovo 2017.
| Trial name or title | Adherence and acceptance of a telemedicine monitoring system for OSA patients treated with CPAP |
| Methods | Randomised, controlled trial |
| Participants | 30 patients (mean age 53.3 ± 9.5 years) with moderate to severe OSA (mean apnoea hypopnoea index (AHI) 45.3 ± 21) that had PAP prescribed |
| Interventions | Standard care (SC) vs telemedicine (TM) |
| Outcomes | % days of use for more than 4 hours at 1, 3 months, reduction in number of visits, patient satisfaction |
| Starting date | 2017 |
| Contact information | |
| Notes | Author correspondence (2 7Feb 2019): results data provided by authors, results have yet to be published as a full manuscript |
Crawford 2016.
| Trial name or title | Evaluating the treatment of obstructive sleep apnoea comorbid with insomnia disorder using an incomplete factorial design |
| Methods | Randomised controlled trial (three‐arm) |
| Participants | N = 140 (expected); males and females age 18 and over will be considered eligible for this study if they meet criteria for ID and OSA. The presence of OSA will be demonstrated by an Apnea‐Hypopnea Index (AHI) = 5 on a full‐night in‐lab baseline polysomnography (PSG) and at least one of the following clinical symptoms: daytime sleepiness or fatigue, unrefreshing sleep, gasping, choking, or holding breath at night, witnessed apneas or loud snoring. Insomnia is characterised by either a complaint of difficulty initiating sleep, maintaining sleep, or waking too early, despite adequate opportunity and circumstances for sleep, coupled with at least one area of significant daytime impairment or distress. In addition, the sleep disturbance will have to be present for at least 3 months. Participants will also have to meet quantitative criteria as evaluated by a sleep diary showing sleep onset latency or wake after sleep onset > 30 minutes, at least 3 nights per week. |
| Interventions | Treatment Arm A: receive sequential treatment beginning with CBT‐I followed by PAP, Treatment Arm B: CBT‐I and PAP are administered concurrently, Treatment Arm C: where individuals receive PAP alone. |
| Outcomes | PSQI, PAP adherence, ISI (Insomnia Severity Index), daytime sleepiness and fatigue, treatment satisfaction |
| Starting date | 2016 |
| Contact information | |
| Notes | Author correspondence (30 Jan 2019): results have yet to be published as a full manuscript |
Kotzian 2018.
| Trial name or title | Home polygraphic recording with telemedicine monitoring for diagnosis and treatment of sleep apnoea in stroke (HOPES Study): study protocol for a single‐blind, randomised controlled trial |
| Methods | Randomised, controlled, single‐blind |
| Participants | 55 patients who had a subacute stroke, aged 19‐70 years, with moderate to severe OSA, who have undergone successful PAP training and titration at the neurorehabilitation unit. |
| Interventions | PAP training strategy during in hospital rehabilitation combined with a telemedicine monitoring system vs standard care |
| Outcomes | CPAP adherence (minutes per night), systolic BP, Barthel Index |
| Starting date | 2016 |
| Contact information | |
| Notes | Results have yet to be published as a full manuscript. |
Seixas 2018.
| Trial name or title | |
| Methods | Randomised parallel‐group trial |
| Participants | N = 398 Patients of African descent with low‐to‐high OSA risk |
| Interventions | Culturally‐tailored peer education/support groups vs. standard care with same level of contact |
| Outcomes | Rate of adherence to recommended home OSA evaluation and treatment, rate of OSA among black men and women at the community level |
| Starting date | |
| Contact information | |
| Notes | Reason for "Awaiting Classification" Status: unable to confirm review inclusion criteria at this time. Results data for this study not currently available |
AHI: apnoea hypopnoea index; BP: blood pressure; CBT: cognitive behavioural therapy; CPAP: continuous positive airway pressure; IQR: interquartile range; OSA: obstructive sleep apnoea; PAP: positive airway pressure; PSQI: Pittsburgh Sleep Quality Index.
Differences between protocol and review
The inclusion criteria of the current review varied slightly from the last publication (Wozniak 2014). Studies explicitly recruiting participants with a diagnosis of central sleep apnoea were excluded, and we elected to allow studies that utilised different makes of CPAP devices provided that proper randomisation was apparent and disproportionate representation of any one CPAP device in a treatment arm was unlikely. Subjective participant reports of the CPAP machine usage were not analysed as studies that included this outcome were not of sufficient quality to be considered meaningfully.
Due to considerable variation in endpoints, we elected to use an endpoint of three months (or the measured endpoint closest to three months) as it was both the modal endpoint across studies and, in our judgement, the most clinically‐relevant among those commonly reported.
'Risk of bias' assessments were conducted using both the previous 'Risk of bias1 ' tool (Higgins 2011) and the newly revised 'Risk of bias 2' tool (Sterne 2019). The 'Risk of bias 2' tool was employed to give an outcome‐level assessment of bias for the review's main outcome of interest (CPAP machine usage). The 'Risk of bias 1' tool was used to give a study‐level assessment of overall bias for all outcomes of interest. These tools were used for all 41 included studies in this review. Information derived from the application of both 'Risk of bias' tools were used in conducting the GRADE assessments; details are provided within the Methods section under 'Summary of findings' tables.
Relative to outcomes, we elected to add proportion of people adherent to CPAP (four hours/night), oxygen desaturation index (ODI), and cost‐effectiveness as secondary outcomes. Due to finding very few, or no studies in the previous review (Wozniak 2014), we excluded the following outcomes from the present review: maintenance of wakefulness, cardiovascular outcomes and adverse events.
For subgroup analyses, we planned to adjust the categorisation of apnoea hypopnoea index (AHI) severity (now mild (AHI ≥ 5 to < 15), moderate (AHI ≥ 15 to < 30), severe (AHI ≥ 30)), and to complete an additional stratification by baseline Epworth Sleepiness Scale score.
Multiple post‐hoc analyses were conducted for the main outcome of CPAP usage that were not previously specified in the protocol. These analyses were conducted to explore the effects of:
intervention classification decisions (updated review versus previous review) (Analysis 5.1, Analysis 5.2, Analysis 5.3);
excluding "high" risk of bias studies, within each class (Analysis 5.4, Analysis 5.5, Analysis 5.6);
grouping studies (within each class) based on intervention duration, number of contact episodes, and total contact time (Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.4, Analysis 6.7; Analysis 6.8; Analysis 6.9; Analysis 6.10; Analysis 6.11; Analysis 6.12);
grouping studies (within the supportive class) based on whether there was human versus automated‐only (Analysis 6.5), and scheduled versus non‐scheduled human support (Analysis 6.6);
our selection of the modal three‐month endpoint (Additional Table 16, summary of analyses).
5.1. Analysis.
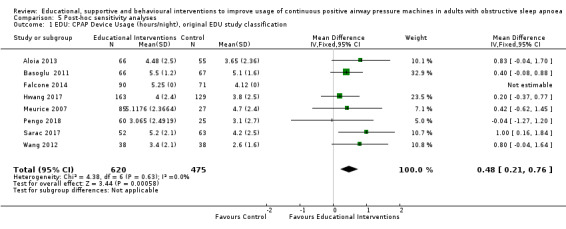
Comparison 5 Post‐hoc sensitivity analyses, Outcome 1 EDU: CPAP Device Usage (hours/night), original EDU study classification.
5.2. Analysis.
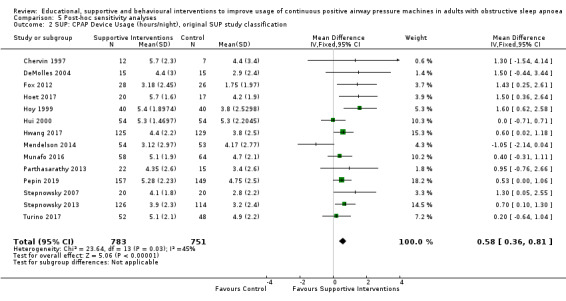
Comparison 5 Post‐hoc sensitivity analyses, Outcome 2 SUP: CPAP Device Usage (hours/night), original SUP study classification.
5.3. Analysis.
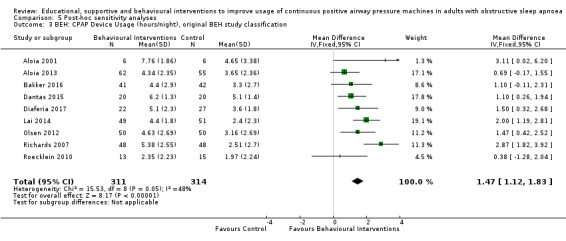
Comparison 5 Post‐hoc sensitivity analyses, Outcome 3 BEH: CPAP Device Usage (hours/night), original BEH study classification.
5.4. Analysis.
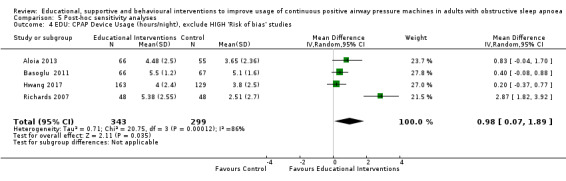
Comparison 5 Post‐hoc sensitivity analyses, Outcome 4 EDU: CPAP Device Usage (hours/night), exclude HIGH 'Risk of bias' studies.
5.5. Analysis.
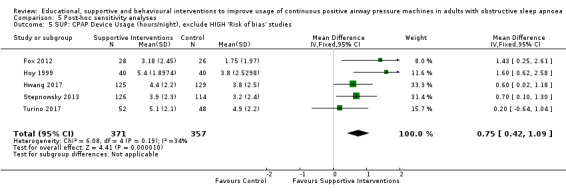
Comparison 5 Post‐hoc sensitivity analyses, Outcome 5 SUP: CPAP Device Usage (hours/night), exclude HIGH 'Risk of bias' studies.
5.6. Analysis.
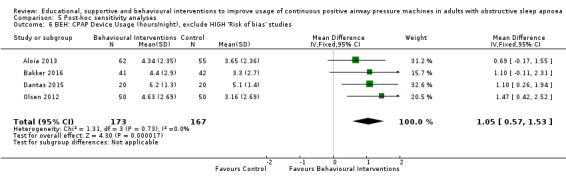
Comparison 5 Post‐hoc sensitivity analyses, Outcome 6 BEH: CPAP Device Usage (hours/night), exclude HIGH 'Risk of bias' studies.
Careful considerations were given regarding the effects of interventions on cardiovascular outcomes related to obstructive sleep apnoea (OSA); these considerations can be found in Appendix 1.
Contributions of authors
KDA: review conception/development, study screening/assessment, data extraction, data entry and analysis, and preparation of report (2019)
LW: review conception/development, study screening/assessment, data extraction, data entry and analysis, and preparation of report (2019)
TE: data extraction, data entry and analysis, and preparation of report (2019)
JC: review conception/development, data extraction (2019).
DRW: review conception/development, study screening/assessment (2013); data extraction (2013), data entry and analysis (2013); and preparation of report (2013 and 2019).
IS: review conception/development, study screening/assessment (2013), data extraction (2013) and analysis (2013), and preparation of report (2009, 2013 and 2019).
Previous author(s) no longer contributing to this version of the review
TJL: Study assessment (2009); data extraction, data entry and analysis (2009); write‐up (2013).
Vidya Nadig (2009): study assessment; data extraction; write‐up.
Contributions of editorial team
Rebecca Fortescue (Co‐ordinating Editor): edited the review; advised on methodology, interpretation and content.
Chris Cates (Co‐ordinating Editor) checked the data entry prior to the full write up of the review; advised on methodology, interpretation and content, approved the final review prior to publication.
Emma Dennett (Managing Editor): co‐ordinated the editorial process; advised on interpretation and content; edited the review.
Emma Jackson (Assistant Managing Editor): conducted peer review; obtained translations; edited the Plain language summary and reference sections of the protocol and the review.
Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the search methods section.
Sources of support
Internal sources
Royal Papworth NHS Trust, UK.
Waypoint Research Institute, Waypoint Centre for Mental Health Care, Canada.
External sources
The authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
K. Askland: none known.
L. Wright: is employed by AstraZeneca Canada. AstraZeneca Canada has no financial interest in the findings of this non‐pharmaceutical review, nor do they have business involvement with CPAP devices or any competing interventions for sleep apnoea.
D. Wozniak: none known.
T. Emmanuel: none known.
J. Caston: none known.
I. Smith: 2018 speakers fee at conference sponsored by Fisher and Paykel (topic aviation medicine). 2018 support for conference attendance Itamar Medical, manufacturer of sleep diagnostic equipment.
These authors contributed equally to this work.
These authors contributed equally to this work.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aloia 2001 {published data only}
- Aloia MS, Dio L, Ilnicky N, Perlis ML, Greenblatt DW, Giles DE. Improving compliance with nasal CPAP in older adults with OAHS. Sleep and Breathing 2001;5(1):13‐21. [DOI] [PubMed] [Google Scholar]
- Aloia MS, Ilnicky N, Dio P, Perlis M, Greenblatt DW, Giles DE. Neuropsychological changes and treatment compliance in older adults. Journal of Psychosomatic Research 2003;54(1):71‐6. [DOI] [PubMed] [Google Scholar]
Aloia 2013 {published data only}
- Aloia MS, Arnedt JT, Strand M, Millman RP, Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: a randomized controlled trial. Sleep 2013;36(11):1655‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia MS, Arnedt JT, Strand M, Millman RP, Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: a randomized controlled trial. Sleep MISC1 ‐ SLP. Darien IL 60561, United States: United States Associated Professional Sleep Societies,LLC, 2012; Vol. 812 0087;36(11):1655‐62 U. [CRSSTD ‐ 4224356] [DOI] [PMC free article] [PubMed]
Bakker 2016 {published data only}
- Bakker JP, Wang R, Weng J, Aloia MC, Toth C, Morrical MG, et al. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: a randomized controlled trial. Chest 2016;150(2):337‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker JP, Wang R, Weng J, Aloia MS, Toth C, Morrical MG, Gleason KJ, et al. Motivational enhancement for increasing adherence to CPAP: a randomized controlled trial. Chest. United States Elsevier B.V., 2016; Vol. 150 MISC1 ‐ SLP(2 AID ‐ S0012‐3692(16)45716‐8):337‐345. [10.1016/j.chest.2016.03.019 CNO ‐ CN‐01139857] [DOI] [PMC free article] [PubMed]
- NCT01261390. Sleep apnea intervention for cardiovascular disease reduction. clinicaltrials.gov/show/NCT01261390 2010. [CRSSTD ‐ 6863291]
- Yaggi HK, Sico J, Concato J, Qin L, Tobias L, Bravata D. Diagnosing and treating sleep apnea in patients with acute cerebrovascular disease: the sleep tight study. Sleep [Conference]. Associated Professional Sleep Societies,LLC, 2013; Vol. 39 MISC1 ‐ SLP():A134. [Ref ID: 26333]
Bartlett 2013 {published data only}
- Bartlett D, Moy E, Wong K, Richards D, Espie C, Cistulli P, et al. Can we increase adherence to CPAP treatment for obstructive sleep apnea (OSA) with a group cognitive behavioural therapy (CBT) treatment intervention: a randomised trial. Conference poster RTY. 2013; Vol. CRSSTD ‐ 4218951:NA. [CRSSTD ‐ 4218951]
- Bartlett D, Wong K, Richards D, Moy E, Espie CA, Cistulli PA, et al. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep 2013;36(11):1647‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DJ, Hoyos CM, Wong KK, Dianne R, Ronald GR, Peter LY. The role of social cognitive theory (SCT) in CPAP adherence: Data from two randomised controlled studies. Sleep and Biological Rhythms. Conference: Worldsleep2011. Blackwell Publishing, 2011; Vol. 9 MISC1 ‐ SLP(4):295.
Basoglu 2011 {published and unpublished data}
- Basoglu OK, Midilli M, Midilli R, Bilgen C. Adherence to continuous positive airway pressure therapy in obstructive sleep apnea syndrome: effect of visual education. Sleep and Breathing 2012;16(4):1193‐200. [DOI] [PubMed] [Google Scholar]
Bouloukaki 2014 {published data only}
- Bouloukaki I, Giannadaki K, Mermigkis C, Mauroudi E, Moniaki V, Siafakas N, et al. Intense versus standard follow up to improve continuous positive airway pressure compliance [Abstract]. European Respiratory Society Annual Congress, 2013 Sept 7‐11. Barcelona, Spain: European Respiratory Society, 2013; Vol. 42 MISC1 ‐ SLP(Suppl 57):739s[P3591]. [DOI] [PubMed]
- Bouloukaki I, Giannadaki K, Mermigkis C, Tzanakis N, Mauroudi E, Moniaki V, et al. Intensive versus standard follow‐up to improve continuous positive airway pressure compliance. European Respritory Journal 2014;44:1262‐74. [DOI] [PubMed] [Google Scholar]
- NCT02016339. Intensive versus standard follow up to improve continuous positive airway pressure (CPAP) compliance. clinicaltrials.gov/ct2/show/NCT02016339 (first received 20 December 2013). [NCT02016339]
Chen 2015 {published data only}
- Chen X, Chen W, Hu W, Huang K, Huang J, Zhou Y. Nurse‐led intensive interventions improve adherence to continuous positive airway pressure therapy and quality of life in obstructive sleep apnea patients. Patient Preference and Adherence 2015;9:1707‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chervin 1997 {published data only}
- Chervin RD, Theut S, Bassetti C, Aldrich MS. Compliance with nasal CPAP can be improved by simple interventions. Sleep 1997;20(4):284‐9. [DOI] [PubMed] [Google Scholar]
Dantas 2015 {published data only}
- Dantas AP, Winck JC, Figueiredo‐Braga M. Adherence to APAP in obstructive sleep apnea syndrome: effectiveness of a motivational intervention. Schlaf & Atmung [Sleep & breathing]. 2014; Vol. 19 MISC1 ‐ SLP(1):327‐34. [DOI] [PubMed]
- Dantas AP, Winck JC, Figueiredo‐Braga M. Adherence to APAP in obstructive sleep apnea syndrome: effectiveness of a motivational intervention. Sleep Breath 2015;19:327‐34. [DOI] [PubMed] [Google Scholar]
DeMolles 2004 {published data only}
- DeMolles DA, Sparrow D, Gottlieb DJ, Friedman R. A pilot trial of a telecommunications system in sleep apnea management. Medical Care 2004;42(8):764‐9. [DOI] [PubMed] [Google Scholar]
Diaferia 2017 {published data only}
- Diaferia G, Santos‐Silva R, Truksinas E, Haddad FL, Santos R, Bommarito S, et al. Myofunctional therapy improves adherence to continuous positive airway pressure treatment. Sleep and Breathing. Springer Verlag, 2016; Vol. MISC2 ‐ 2016‐12:1‐9. [DOI] [PubMed]
- Diaféria G, Santos‐Silva R, Truksinas E, Haddad FL, Santos R, Bommarito S, et al. Myofunctional therapy improves adherence to continuous positive airway pressure treatment. Sleep and Breathing 2017;21(2):387‐95. [DOI] [PubMed] [Google Scholar]
Falcone 2014 {published data only}
- Falcone VA, Damiani MF, Quaranta VN, Capozzolo A, Resta O. Polysomnograph chart view by patients: a new educational strategy to improve CPAP adherence in sleep apnea therapy. Respiratory Care 2014;59(2):193‐8. [DOI] [PubMed] [Google Scholar]
Fox 2012 {published data only}
- Fox N, Hirsch‐Allen A, Goodfellow E, Wenner J, Fleetham JA, Ryan C, et al. Can a telemedicine monitoring system improve CPAP adherence In patients with obstructive sleep apnea (OSA)? [Abstract]. American Journal of Respiratory and Critical Care Medicine. American Thoracic Society, 2010; Vol. 181 MISC1 ‐ SLP (1 MeetingAbstracts):A5570.
- Fox N, Hirsch‐Allen AJ, Goodfellow E, Wenner J, Fleetham JA, Ryan CF, et al. The impact of a telemedicine monitoring system on positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Sleep 2012;35(4):477‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00561860. Use of a remote‐monitoring system to diagnose and treat obstructive sleep apnea. clinicaltrials.gov/show/NCT00561860 21 November 2007.
Hoet 2017 {published data only}
- Hoet F, Libert W, Sanida C, Broecke S, Bruyneel AV, Bruyneel M. Telemonitoring in continuous positive airway pressure‐treated patients improves delay to first intervention and early compliance: a randomized trial. Sleep Medicine 2017;39:77‐83. [DOI] [PubMed] [Google Scholar]
- NCT02773953. Impact of telemonitoring to improve adherence in continuous positive airway pressure (CPAP)‐treated patients. clinicaltrials.gov/ct2/show/NCT02773953 (first received 16 May 2016). [NCT02773953]
Hoy 1999 {published data only}
- Hoy CJ, Vennelle M, Kingshott RN, Engleman HM, Douglas NJ. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome?. American Journal of Respiratory and Criticalcal Care Medicine 1999;159(4):1096‐100. [DOI] [PubMed] [Google Scholar]
Hui 2000 {published data only}
- Hui DS, Chan JK, Choy DK, Ko FW, Li TS, Leung RC, et al. Effects of augmented continuous positive airway pressure education and support on compliance and outcome in a Chinese population. Chest 2000;117(5):1410‐5. [DOI] [PubMed] [Google Scholar]
Hwang 2017 {published data only}
- Chang J, Derose S, Benjafield A, Crocker M, Kim J, Becker K, et al. Acceptance and impact of telemedicine in patient sub‐groups with obstructive sleep apnea: analysis from the tele‐OSA randomized clinical trial. Sleep. Conference: 31st anniversary meeting of the associated professional sleep societies, LLC, SLEEP. Netherlands Associated Professional Sleep Societies,LLC, 2017; Vol. 40 MISC1 ‐ SLP():A201‐A202. [CRSSTD ‐ 6863244 DBP ‐ 201723 ]
- Chang J, Kim J, Becker K, Benjafield A, Crocker M, Woodrum R, et al. Impact of automated web‐education and CPAP tele‐monitoring on CPAP adherence at 3 months and 1 year: the tele‐OSA randomized clinical trial. Sleep. Conference: 31st anniversary meeting of the associated professional sleep societies, LLC, SLEEP. Netherlands Associated Professional Sleep Societies,LLC, 2017; Vol. 40 MISC1 ‐ SLP():A189‐A190.
- Chang J, Liang J, Becker KA, Kim JB, Woodrum R, Vega DT, et al. Impact of interactive web‐based education and automated feedback program on CPAP adherence for the treatment of obstructive sleep apnea. Sleep. Associated Professional Sleep Societies,LLC, 2016; Vol. 39 MISC1 ‐ SLP():A386.
- Crocker M, Chang J, Liang J, Becker K, Kim J, Woodrum R, et al. CRSSTD ‐ 6863244Crocker M; Chang J; Liang J; Becker Automated Tele‐Monitoring on CPAP Adherence at 1 Year: the Tele‐OSA Trial. European Respiratory journal. 2017; Vol. 50 MISC1 ‐ SLP(suppl 61):PA4729. [CRSSTD ‐ 6863244]
- Hwang D, Chang J, Liang J, Becker K, Kim JB, Woodrum RR, et al. Impact of Interactive web‐based education and automated feedback program on CPAP adherence for the treatment of obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine. 2016; Vol. 193 MISC1 ‐ SLP(Meeting Abstracts):A4186.
- Hwang D, Chang JW, Benjafield AV, Crocker ME, Kelly C, Becker KA, et al. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence the Tele‐OSA Randomized Trial. American Journal of Respiratory and Critical Care Medicine. United States American Thoracic Society, 2018; Vol. 197 MISC1 ‐ SLP(1):117‐26. [CRSSTD ‐ 6863244 DBP ‐ 2017/09/01 06:00] [DOI] [PubMed]
- Hwang D, Chang JW, Benjafield AV, Crocker ME, Kelly C, Becker KA, et al. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence. The Tele‐OSA randomized trial. American Journal of Respiratory and Critical Care Medicine 2017;197(1):117‐26. [DOI] [PubMed] [Google Scholar]
- ISRCTN12865936. The effect of telemonitoring on adherence to continuous positive airway pressure in sleep apnea hypopnea syndrome. www.isrctn.com/ISRCTN12865936 (first received 18 January 2017).
Lai 2014 {published data only}
- Lai A, Lam J, Fong D, Ip M. Long‐term efficacy of motivational interviewing on improving continuous positive airway pressure adherence in obstructive sleep apnea: A randomized controlled trial [Abstract]. European Respiratory Society Annual Congress, 2013 Sept 7‐11. Barcelona, Spain: European Respiratory Society, 2013; Vol. 1142 MISC1 ‐ SLP(Suppl 57):740 s [P3595].
- Lai AY, Fong DY, Lam JC, Weaver TE, Ip MS. The efficacy of a brief motivational enhancement education program on CPAP adherence in OSA: a randomized controlled trial.. Chest 2014;146(3):600‐10. [DOI] [PubMed] [Google Scholar]
- NCT01173406. Education programme on continuous positive airway pressure treatment. clinicaltrials.gov/ct2/show/NCT01173406 (first received 2 August 2010). [NCT01173406]
- NCT01428921. Long term efficacy of education programme on continuous positive airway pressure treatment. clinicaltrials.gov/ct2/show/NCT01428921 (first received 5 September 2011).
Lewis 2006 {published data only}
- Lewis KE, Bartle IE, Watkins AJ, Seale L, Ebden P. Simple interventions improve re‐attendance when treating the sleep apnoea syndrome. Sleep Medicine 2006;7(3):241‐7. [DOI] [PubMed] [Google Scholar]
Mendelson 2014 {published data only}
- Mendelson M, Vivodtzev I, Taminiser R, Laplaud D, Dias‐Domingos S, Baguet J‐P, et al. Continuous positive airway pressure (CPAP) supported by telemedicine improves sleepiness and quality of life but not blood pressure in high cardiovascular risk obstructive sleep apnea (OSA): A randomized, controlled trial [Abstract]. European Respiratory Society Annual Congress. European Respiratory Society, 2013; Vol. 42 MISC1 ‐ SLP(Suppl 57):406s [P2044].
- Mendelson M, Vivodtzev I, Tamisier R, Laplaud D, Dias‐Domingos S, Baguet JP, et al. CPAP treatment supported by telemedicine does not improve blood pressure in high cardiovascular risk OSA patients: a randomized, controlled trial. Sleep 2014;37(11):1863‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meurice 2007 {published data only}
- Meurice JC, Ingrand P, Portier F, Arnulf I, Rakotonanahari D, Fournier E, et al. The ANTADIR Working Group "PPC", CMTS ANTADIR. A multicentre trial of education strategies at CPAP induction inthe treatment of severe sleep apnoea–hypopnoea syndrome. Sleep Medicine 2007;8(1):37‐42. [DOI: 10.1016/j.sleep.2006.05.010] [DOI] [PubMed] [Google Scholar]
Munafo 2016 {published data only}
- Munafo D, Hevener W, Crocker M, Willes L, Sridasome S, Muhsin M. A telehealth program for CPAP adherence reduces labor and yields similar adherence and efficacy when compared to standard of care. Sleep and Breathing 2016;20(2):777‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo D, Hevener W, Sridasome S, Gansevich B, Crocker M, Willes L, et al. A web based automated messaging program for CPAP adherence coaching reduced the coaching labor required while yielding similar adherence and efficacy to standard of care coaching (Abstract). American Journal of Respiratory and Critical Care Medicine. American Thoracic Society, 2014; Vol. 189 MISC1 ‐ SLP():A6570.
Olsen 2012 {published data only}
- Olsen S, Smith S, Oei T, Douglas J. Motivational Interviewing (MINT) improves continuous positive airway pressure (CPAP) acceptance and adherence: a randomised controlled trial. Journal of Consulting and Clinical Psychology 2012;80(1):151‐63. [DOI] [PubMed] [Google Scholar]
- Olsen S, Smith S, Oei TP, Douglas J. Motivational intervention improves CPAP acceptance and adherence. Sleep and Biological Rhythms. 2009; Vol. 7 MISC1 ‐ SLP(Suppl 1):A202009.
Parthasarathy 2013 {published and unpublished data}
- NCT01164683. A pilot study of CPAP adherence promotion by peer buddies with sleep apnea (CPAPPs). clinicaltrials.gov/ct2/show/NCT01164683 (first received 19 July 2010).
- Parthasarathy S, Wendel C, Haynes PL, Atwood C, Kuna ST. A pilot study of CPAP adherence promotion by peer buddies with sleep apnea. American Journal of Respiratory and Critical Care Medicine CNO ‐ CN‐01091745. American Thoracic Society, 2012; Vol. 185:NA. [DOI] [PMC free article] [PubMed]
- Parthasarathy S, Wendel C, Haynes PL, Atwood C, Kuna ST. A pilot study of CPAP adherence promotion by peer buddies with sleep apnea. Journal of Clinical Sleep Medicine 2013;9(6):543‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S, Wendel C, Haynes PL, Atwood C, Kuna ST. A pilot study of CPAP adherence promotion by peer buddies with sleep apneaAmerican journal of respiratory and critical care medicine. SLP(Meeting Abstracts). American Thoracic Society, 2012; Vol. 185 MISC1‐SLP (Meeting Abstracts):A3845.
Pengo 2018 {published data only}
- Berry M, Czaban M, Pengo M, Nirmalan P, Steier J. The effect of positive and negative message framing on continuous positive airway pressure (CPAP) therapy adherence in patients with obstructive sleep apnoea [Abstract]. European Respiratory Journal. 2014; Vol. 44 MISC1 ‐ SLP(Suppl 58):P49192014.
- Pengo MF, Czaban M, Berry MP, Nirmalan P, Brown R, Birdseye A, et al. The effect of positive and negative message framing on short term continuous positive airway pressure compliance in patients with obstructive sleep apnea. Journal of Thoracic Disease 2018;10(Suppl 1):S160‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pepin 2019 {published data only}
- NCT01226641. Usefulness of a telemedicine system for OSA patients follow‐up with high cardiovascular risk (TELESAS). clinicaltrials.gov/show/NCT01226641 2010.
- Pepin J, Sapene M, Benmerad M, Bailly S, Grillet Y, Stach B, et al. Effectiveness of multimodal remote patient monitoring at CPAP initiation in obstructive sleep apnea (OSA) with high cardiovascular risk: a randomized trial DBP ‐ 201830. American Journal of Respiratory and Critical Care Medicine CNO ‐ CN‐01619216. Netherlands American Thoracic Society RTY, 2018; Vol. 197(Meeting Abstracts):A746. [CRSSTD ‐ 10905747]
- Pepin J‐L, Jullian‐Desayes I, Sapene M, Treptow E, Joyeux‐Faure M, Benmerad M, et al. Multimodal remote monitoring of high cardiovascular risk OSA patients initiating CPAP: a randomized trial. Chest. United States Elsevier Inc, 2019; Vol. 155(4):730‐9. [CRSSTD ‐ 10905822] [DOI] [PubMed]
- Pepin J‐L, Mendelson M, Vivodtzev I, Tamisier R, Laplaud D, Dias Domingos S, et al. Continuous positive airway pressure (CPAP) supported by telemedicine improves sleepiness and quality of life but not blood pressure in high cardiovascular risk obstructive sleep apnea (OSA): A randomized, controlled trial. American Journal of Respiratory and Critical Care Medicine. American Thoracic Society, 2014; Vol. 189 MISC1 ‐ SLP():A5626.
- Pépin JL, Jullian‐Desayes I, Sapène M, Treptow E, Joyeux‐Faure M, Benmerad M, et al. Multimodal remote monitoring of high cardiovascular risk patients with OSA initiating CPAP: a randomized trial. Chest 2019;155(4):730‐9. [DOI] [PubMed] [Google Scholar]
Richards 2007 {published data only}
- Richards D, Bartlett DJ, Wong K, Malouff J. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep 2007;30(5):635‐40. [DOI] [PubMed] [Google Scholar]
Roecklein 2010 {published data only}
Sarac 2017 {published data only}
- Saraç S, Afşa GC, Oruç Ö, Topçuoğlu OB, Saltürk C, Peker Y. Impact of patient education on compliance with positive airway pressure treatment in obstructive sleep apnea. Medical Science Monitor 2017;23:1792‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawyer 2017 {published data only}
- Sawyer AM, King TS, Weaver TE, Sawyer DA, Varasse M, Franks J, et al. A tailored intervention for PAP adherence: the SCIP‐PA trial. Behavioral Sleep Medicine 2017;17(1):49‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer AM, Sawyer DA, King TS, Weaver TE, Varrasse M, Franks J, et al. Complex tailored intervention pilot randomized controlled trial: social cognitive intervention to promote positive airway pressure (PAP) adherence ‐‐ the SCIP‐PA trial...28th annual scientific session. Nursing Research. Baltimore, Maryland: Lippincott Williams & Wilkins, 2016; Vol. 65(2 MISC1 ‐ SLP):E52‐E53. [CRSSTD ‐ 9291866]
Scala 2012 {published data only}
- Scala D, Starace A, Lembo L, Falco F, Niola M, Lisi R, et al. Therapeutic patient education program for patient with obstructive sleep apnea syndrome (OSAS): preliminary results. Bollettino della Società Italiana di Farmacia Ospedaliera 2012;59(5):195‐201. [Google Scholar]
Sedkaoui 2015 {published data only}
- Didier A, Leseux L, Sedkaoui K, Pontier S, Adrover L, Fraysse JL. Education of patients with sleep apnea syndrome: feasibility of a phone coaching procedure. European Respiratory Journal. European Respiratory Society, 2011; Vol. 38 MISC1 ‐ SLP(Suppl 55):321.
- Sedkaoui K, Leseux L, Pontier S, Rossin N, Leophonte P, Fraysse JL, et al. Efficiency of a phone coaching program on adherence to continuous positive airway pressure in sleep apnea hypopnea syndrome: a randomized trial. BMC Pulmonary Medicine 2015;15(102):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedkaoui K, Leseux L, Pontier S, Rossin N, Leophonte P, Fraysse JL, Didier A. Efficiency of a phone coaching program on adherence to continuous positive airway pressure in sleep apnea hypopnea syndrome: a randomized trial. BMC Pulmonary Medicine. England BioMed Central Ltd, 2015; Vol. 15 MISC1 ‐ SLP():102. [DOI] [PMC free article] [PubMed]
- Sedkaoui K, Pontier S, Rossin N, Leseux L, Adrover L, Leophonte P, et al. Education of patients with sleep apnea‐hypopnea syndrome: Efficiency of a phone coaching procedure [Abstract]. European Respiratory Society Annual Congress, MISC1 ‐ SLP(Suppl 57). Barcelona, Spain: European Respiratory Society, 2013; Vol. 42 MISC1‐SLP(Suppl 57):717s [3504].
Shapiro 2017 {published data only}
- Shapiro AL. Effect of the CPAP‐SAVER Intervention on Adherence Among Adults with Newly Diagnosed Obstructive Sleep Apnea (Unpublished doctoral dissertation). ProQuest Publications 2017. [ProQuest ID: 10266828]
Smith 2006 {published data only}
- Smith CE, Dauz ER, Clements F, Puno FN, Cook D, Doolittle G, et al. Telehealth services to improve non‐adherence: a placebo‐controlled study. Telemedicine Journal and E‐Health 2006;12(3):289‐96. [DOI] [PubMed] [Google Scholar]
Smith 2009 {published data only}
- Smith CE, Dauz E, Clements F, Werkowitch M, Whitman R. Patient education combined in a music and habit‐forming intervention for adherence to continuous positive airway (CPAP) prescribed for sleep apnea. Patient Education and Counseling 2009;74(2):184‐90. [0738‐3991] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soares‐Pires 2013 {published data only}
- Soares Pires F, Drummond M, Marinho A, Sampaio R, Pinto T, Gonçalves M, et al. Effectiveness of a group education session on adherence with APAP in obstructive sleep apnea‐ a randomized controlled study. Sleep and Breathing 2013;17(3):993‐1001. [DOI] [PubMed] [Google Scholar]
Sparrow 2010 {published data only}
- NCT00232544. Telecommunications system in sleep apnea. clinicaltrials.gov/ct2/show/NCT00232544 (first received 4 October 2005.
- NCT01715194. Telemedicine to enhance adherence to CPAP therapy in patients with OSAS. linicaltrials.gov/ct2/show/NCT01715194.
- Sparrow D, Aloia M, Demolles DA, Gottlieb DJ. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomised controlled trial. Thorax 2010;65(12):1061‐6. [DOI] [PubMed] [Google Scholar]
Stepnowsky 2007 {published data only}
- Stepnowsky C, Palau JJ, Marler MM, Gifford AL. Effect of wireless monitoring on CPAP adherence and treatment efficacy: a pilot study [Abstract]. Sleep. 2006; Vol. 29 MISC1 ‐ SLP(Suppl):A173. [Stepnowsky 2007]
- Stepnowsky CJ, Palau JJ, Marler MR, Gifford AL. Pilot randomized trial of the effect of wireless telemonitoring on compliance and treatment efficacy in obstructive sleep apnea. Journal of Medical Internet Research 2007;9(2):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepnowsky CJ, Palau JJ, Zamora T, Marler M. Effect of wireless monitoring on CPAP adherence and treatment efficacy. Telemedicine and E‐Health 2008;14((Suppl 1)):25. [Google Scholar]
Stepnowsky 2013 {published data only}
- Stepnowsky C, Edwards C, Zamora T, Barker R, Agha Z. Patient perspective on use of an interactive website for sleep apnea. International Journal of Telemedicine and Applications 2013;2013:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepnowsky C, Edwards C, Zamora T, Agha Z, Ancoli‐Israel S, Loredo J. Effect of an internet intervention on CPAP adherence [Abstract]. Sleep. 2013; Vol. 34 MISC1 ‐ SLP MISC2 ‐ 6/12:A120[0341].
Turino 2017 {published data only}
- NCT02517346. Efficacy of telemonitoring on CPAP treatment compliance in obstructive sleep apnea (OSA) patients. clinicaltrials.gov/show/NCT02517346 (first received 7 April 2015).
- Turino C, Batlle J, Woehrle H, Mayoral A, Castro A, Gomez S, et al. Management of CPAP treatment compliance using telemonitoring in obstructive sleep apnoea. American Journal of Respiratory and Critical Care Medicine. Netherlands American Thoracic Society, 2017; Vol. 195 MISC1 ‐ SLP(Meeting Abstracts):A6540.
- Turino C, Batlle J, Woehrle H, Mayoral A, Castro‐Grattoni AL, Gómez S, et al. Management of continuous positive airway pressure treatment compliance using telemonitoring in obstructive sleep apnoea. European Respiratory Journal 2017;49(2):1‐8. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang W, He G, Wang M, Liu L, Tang H. Effects of patient education and progressive musclerelaxation alone or combined on adherence to continuous positive airway pressure treatment in obstructive sleep apnea patients. Sleep & Breathing 2012;16(4):1049‐1057. [DOI: 10.1007/s11325-011-0600-3] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aloia 2005 {published data only}
- Aloia MS, Arnedt J, Zimmerman M, Skrekas J, Harris S, Carlise C, et al. Combined therapy to improve adherence to CPAP. Sleep 2005;28:A192. [Google Scholar]
Andreu 2012 {published data only}
- Andreu AL, Chiner E, Sancho‐Chust JN, Pastor E, Llombart M, Gomez‐Merino, et al. Effect of an ambulatory diagnostic and treatment programme in patients with sleep apnoea. European Respiratory Journal 2012;39:305‐12. [DOI] [PubMed] [Google Scholar]
Andrews 2010 {published data only}
- Andrews ND, Auckley D, Benca R, Iber C, Kapur VK, Redline S, et al. The impact of PAP education and troubleshooting on 3‐month adherence in subjects with obstructive sleep apnea (OSA) participating in the HomePAP study [Abstract]. Sleep 2010;33(Supp):A167. [Google Scholar]
Antic 2009 {published data only}
- Antic NA, Buchan C, Esterman A, Hensley M, Naughton MT, Rowland S, et al. A randomized controlled trial of nurse‐led care for symptomatic moderate‐severe obstructive sleep apnea. American Journal ofRespiratory and Critical Care Medicine 2009;179(6):501‐8. [DOI] [PubMed] [Google Scholar]
Arrua 2016 {published data only}
- Arrua VM, Garmendia O, Moraleda A, Farre R, Guerrero G, Ruiz C, et al. Applicability, efficacy and cost effectiveness of sleep apnoea management by an information and communication based technology (ICT). Rationale, design and methods. European Respiratory Journal 2016;48:PA2375. [Google Scholar]
Bague 2015 {published and unpublished data}
- Bague A. Pneumotoning (oropharyngeal and pulmonary exercises, electrical stimulation and manual therapy) to improve the CPAP compliance in patients with obstructive sleep apnea‐hypopnea. A pilot study. Somnologie 2015;19(SUPPL. 1):38. [Google Scholar]
Bague‐Cruz 2014 {published and unpublished data}
- Bague‐Cruz A, Esteller E. Pneumotoning (oropharyngeal and pulmonary exercises, electrical stimulation and manual therapy) to improve the continuous positive airway pressure's compliance in patients with obstructive sleep apnoea‐hypopnoea. A pilot study. European Respiratory Journal 2014;58:4678. [Google Scholar]
Baltzan 2014 {published data only}
- Baltzan M, Capozzolo B, Champagne K, Tanzimat G, Wolkove N, Verschelden P. Final results of a double‐blind, randomized, parallel group trial of initial zopiclone prescription on the adherence with continuous positive airway pressure (CPAP) in adults treated for obstructive sleep apnea (OSA). American Journal of Respiratory and Critical Care Medicine 2014;189:A3669. [Google Scholar]
Barbe 2013 {published and unpublished data}
- NCT01918449. Follow‐up of patients with obstructive sleep apnea in primary care. clinicaltrials.gov/ct2/show/NCT01918449 (first received 7 August 2013).
Barbe 2014 {published data only}
- Barbe F, Esquinas C, Nadal N, Serra S, Pascual L, Cortijo A, et al. Primary care vs specialist sleep center management of continuous positive airway pressure treatment in obstructive sleep apnea patients. A randomized controlled trial (Abstract). American Journal of Respiratory and Critical Care Medicine 2014;189:A3671. [Google Scholar]
Berry 2008 {published data only}
- Berry RB, Hill G, Thompson L, McLaurin V. Portable monitoring and autotitration versus polysomnography for the diagnosis and treatment of sleep apnea. Sleep 2008;31(10):1423‐31. [PMC free article] [PubMed] [Google Scholar]
Billings 2010 {published data only}
- Billings ME, Auckley D, Benca R, Foldvary‐Schaefer N, Iber C, Redline S, et al. Race and education as predictors of CPAP adherence [Abstract]. Sleep 2010;33:A122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bittencourt 2015 {published data only}
- Bittencourt L, Servante D, Javaheri S, Tufik S. Effects of exercise training and CPAP in patients with heart failure and obstructive sleep apnea. Sleep 2015;38:A177. [Google Scholar]
Cartwright 2017 {published and unpublished data}
- Cartwright A, Depew A, Burleson A, Vannoni V, Simmons B, Goelz K, et al. Use of a personalized video to enhance PAP adherence: preliminary report from a randomized clinical trial. Sleep 2017;40:A190. [Google Scholar]
Chai‐Coetzer 2011 {published data only}
- Chai‐Coetzer CL, Antic N, Rowland LS, Reed R, Esterman A, Vowles N, et al. A randomised controlled trial to evaluate a simplified model of care for obstructive sleep apnea in general practice. Journal of Sleep Research 2011;20(Supp 1):14. [Google Scholar]
Chai‐Coetzer 2012 {published data only}
- Chai‐Coetzer CL, Antic NA, Rowland LS, Reed RL, Esterman A, Catcheside P. A randomised controlled trial to evaluate a simplified model of care for obstructive sleep apnea in primary care [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185:A3853. [Google Scholar]
Chai‐Coetzer 2013 {published data only}
- Chai‐Coetzer CL, Antic NA, Rowland LS, Reed RL, Esterman A, Catcheside PG, et al. Primary care vs specialist sleep center management of obstructive sleep apnea and daytime sleepiness and quality of life: a randomized trial. JAMA 2013;309(10):997‐1004. [DOI] [PubMed] [Google Scholar]
Cotton 2012 {published data only}
- Cotton J, Zarrouf FA. Weekly text messaging to improve CPAP compliance: A randomized prospective trial. Sleep 2012;35:A165. [Google Scholar]
Damjanovic 2005 {published data only}
- Damjanovic D, Fluck A, Idzko M, Muller‐Quernheim J, Sorichter S. Nasal CPAP in the therapy of OSAS ‐ does a closer patient guidance and support increase compliance? [Abstract]. European Respiratory Journal 2005;26(Supp 49):A4521. [Google Scholar]
Damjanovic 2009 {published data only}
- Damjanovic D, Fluck A, Bremer H, Muller‐Quernheim J, Idzko M, Sorichter S. Compliance in sleep apnoea therapy: influence of home care support and pressure mode. European Respiratory Journal 2009;33(4):804‐11. [DOI] [PubMed] [Google Scholar]
Dawson 2015 {published data only}
- Dawson JD, Yu L, Aksan NS, Tippin J, Rizzo M, Anderson SW. Feedback from naturalistic driving improves treatment compliance in drivers with obstructive sleep apnea. Proceeding of the International Driving Symposium on Human Factors in Driver Assessment Training and Vehicle Design 2015;Jun:30‐5. [PMC free article] [PubMed] [Google Scholar]
Deng 2013 {published data only}
- Deng T, Wang Y, Sun M, Chen B. Stage‐matched intervention for adherence to CPAP in patients with obstructive sleep apnea: A randomized controlled trial. Sleep and Breathing Abstract Supp 2013;2:791‐801. [DOI] [PubMed] [Google Scholar]
Di Elia 2008 {published data only}
- Elia CA, Bittencourt LR, Truksinas E, Mello MT, Sousa BS, Silva AC, et al. Effects of exercise training associated with CPAP in moderate to severe obstructive sleep apnea patients [Abstract]. Abstracts of the 22nd Annual Meeting of the Associated Professional Sleep Societies, LLC 2012;31:A183. [Google Scholar]
Engleman 1993 {published data only}
- Engleman HM, Douglas NJ. CPAP compliance. Sleep 1993;16(Supp 8):s114. [DOI] [PubMed] [Google Scholar]
Epstein 2000 {unpublished data only}
- Epstein L, Graham L Turner A, Larkin E, Garshick E, Ayas N, et al. Comparison of two methods for achieving CPAP compliance. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A358. [Google Scholar]
Escourrou 2012 {published data only}
- Escourrou P, Durand‐Zaleski I, Charrier N, Agostini H, Alfandary D, Orvoen‐Frija E, et al. Respiradom: a telemedicine system for the follow‐up of patients with Sleep Apnea Syndrome (abstract). Journal of Sleep Research 2012;21:50. [Google Scholar]
Fields 2016 {published data only}
- Fields BG, Behari PP, McCloskey S, True G, Richardson D, Thomasson A, et al. Remote ambulatory management of Veterans with obstructive sleep apnea. Journal of Sleep and Sleep Disorders Research 2016;39(3):501‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fletcher 1991 {published data only}
- Fletcher EC, Luckett RA. The effect of positive reinforcement on hourly compliance in nasal continuous positive airway pressure users with obstructive sleep apnea. American Review of Respiratory Diseases 1991;143(5 Pt 1):936‐41. [DOI] [PubMed] [Google Scholar]
Fresnelli 2016 {published data only}
- Frasnelli M, Baty F, Niedermann J, Brutsche MH, Schoch OD. Effect of telemetric monitoring in the first 30 days of continuous positive airway pressure adaptation for obstructive sleep apnoea syndrome ‐ a controlled pilot study. Journal of Telemedicine and Telecare 2016;22(4):209‐14. [DOI] [PubMed] [Google Scholar]
Gupta 2011 {published data only}
- Gupta S, Bollavaram N, Knapik S. At home patient directed daytime continuous positive airway pressure (CPAP) mask acclimatization prior to starting CPAP therapy. Chest 2011;140(4):824A. [Google Scholar]
Ha 2015 {published data only}
- Ha S, Lee D, Abdullah V, Hasselt C. Compliance improvement to use CPAP for patients with obstructive sleep apnea diagnosed by portable device. Sleep Medicine 2015;16(Supp 1):S128‐9. [Google Scholar]
Harris 2014 {published data only}
- Harris DL, Nielsen DB, Densley A, Caldwell M, Muhlestein J, Bradshaw D. CPAP compliance > 4 hours per night in the CPAP utilization development from directed learning, education and supervision (CUDDLES) study. Sleep 2014;37:A119‐120. [Google Scholar]
Hayes 2009 {published data only}
- Hayes A, Mehra R. Predictors of continuous positive airway pressure adherence in a randomized controlled trial [Abstract]. Sleep 2009;32:A202. [Google Scholar]
Hood 2013 {published data only}
- Hood MM, Corsica J, Cvengros J, Wyatt J. Impact of a brief dietary self‐monitoring intervention on weight change and CPAP adherence in patients with obstructive sleep apnea. Journal of Psychosomatic Research 2013;74(2):170‐4. [DOI] [PubMed] [Google Scholar]
Hostler 2014 {published data only}
- Hostler J, Sheikh K, Khramtsov A, Andrada T, Holley A. An educational smart phone application improves CPAP adherence. Sleep 2014;37:A106‐7. [Google Scholar]
Hostler 2017 {published data only}
- Hostler JM, Sheikh KL, Andrada TF, Khramtsov A, Holley PR, Holley AB. A mobile, web‐based system can improve positive airway pressure adherence. Journal of Sleep Research 2017;26(2):139‐46. [DOI] [PubMed] [Google Scholar]
Hwang 2014 {published data only}
- Hwang D, Becker K, Adenuga O, Vega D, Chang N, Farooqi S, et al. Impact of automated educational and followup mechanisms on patient engagement in the management of obstructive sleep apnea. Sleep 2014;37:A384. [Google Scholar]
Igelstrom 2018 {published data only}
- Igelstrom H, Asenlof P, Emtner M, Lindberg E. Improvement in obstructive sleep apnea after a tailored behavioural sleep medicine intervention targeting healthy eating and physical activity: a randomised controlled trial. Sleep and Breathing 2018;22(3):653‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelstrom H, Emtner M, Asenlof P, Lindberg E. Improvement in obstructive sleep apnea syndrome after a tailored behavioural medicine intervention targeting healthy eating and physical activity. European respiratory journal. 2016; Vol. 48:NA.
Ip‐Buting 2017 {published data only}
- Ip‐Buting A, Kelly J, Santana MJ, Penz ED, Flemons WW, Tsai WH, et al. Evaluation of an alternative care provider clinic for severe sleep‐disordered breathing: a study protocol for a randomised controlled trial. BMJ Open 2017;7(3):e014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Isetta 2014a {published and unpublished data}
- Isetta V, Leon C, Embid C, Duran‐Cantolla J, Campos‐Rodriguez F, Galdeano M, et al. Telemedicine‐based strategy in the management of sleep apnea: a multicenter randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2014;189:A6358. [Google Scholar]
Isetta 2014b {unpublished data only}
- Isetta V, Leon C, Embid C, Pena M, Cambrodi R, Puertas FJ, et al. Telemedicine‐based strategy for sleep apnea management: A multicenter randomized controlled trial [Abstract]. European Respiratory Journal 2014;Supp 58:P2020. [Google Scholar]
Isetta 2015 {published data only}
- Isetta V, Negrín MA, Monasterio C, Masa JF, Feu N, Álvarez A, et al. A Bayesian cost‐effectiveness analysis of a telemedicine‐based strategy for the management of sleep apnoea: a multicentre randomised controlled trial. Thorax 2015;70(11):1054‐61. [DOI] [PubMed] [Google Scholar]
- NCT01716676. Telemedicine in sleep breathing disorders: a multicenter study. clinicaltrials.gov/show/nct01716676 (firsts received 30 October 2012).
Jones 2016 {published data only}
- Jones B, Nielsen DB, Woodward W, Harris D. CPAP utilization development from directed learning, education and supervision (CUDDLES) study. Sleep 2016;39:A385. [Google Scholar]
Jurado‐Gamez 2015 {published data only}
- Jurado‐Gamez B, Bardwell WA, Cordova‐Pacheco LJ, Garcia‐Amores M, Feu‐Collado N, Buela‐Casal G. A basic intervention improves CPAP adherence in sleep apnoea patients: a controlled trial. Sleep and Breathing 2015;19(2):509‐14. [DOI] [PubMed] [Google Scholar]
Kataria 2017 {published data only}
- Kataria LV, Sundahl CA, Skalina LM, Shah M, Pfeiffer MH, Balish MS, et al. ANNIE: the veterans health administration's personalized text message application promotes compliance with positive airway pressure. SleepAbstract Supp 2017;40:A198. [Google Scholar]
- Kataria LV, Sundahl CA, Skalina LM, Shah M, Pfeiffer MH, Balish MS, et al. Text message reminders and intensive education improves positive airway pressure compliance and cognition in veterans with traumatic brain injury and obstructive sleep apnea: annie pilot study. Sleep 2017;40:A198. [Google Scholar]
Klein 2010 {published data only}
- Klein C, Damjanovic D, Idzko M, Seuthe B, Walterspacher S, Kabitz H, et al. Compliance in sleep apnoea therapy: Influence of home care support and pressure mode ‐ A 5‐year follow‐up [Abstract O98]. Journal of Sleep Research 2010;19(Supp 2):20. [Google Scholar]
Kreutzer 2003 {published data only}
- Kreutzer ML, Guilleminault C. Adherence to nasal continuous positive airway pressure (CPAP) in patients with congestive heat failure[Abstract]. Sleep 2003;26:A260. [Google Scholar]
Kuna 2011 {published data only}
- Kuna ST, Gurubhagavatula I, Maislin G, Hin S, Hartwig KC, McCloskey S, et al. Non inferiority of functional outcome in ambulatory management of obstructive sleep apnea. American Journal of Respiratory CriticalCare Medicine 2011;183(9):1238‐44. [DOI] [PubMed] [Google Scholar]
- NCT00880165. Cost effectiveness of ambulatory management for veterans with sleep apnea. clinicaltrials.gov/show/nct00880165 (first received 13 April 2009).
Kuna 2015 {published data only}
- Kuna ST, Shuttleworth D, Chi Luqi, Schutte‐Rodin S, Friedman E, Guo H, et al. Web‐based access to positive airway pressure usage with or without an initial financial incentive improves treatment use in patients with obstructive sleep apnea. Sleep 2015;38(8):1229‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01335854. Promoting continuous positive airway pressure (CPAP) adherence. clinicaltrials.gov/ct2/show/NCT01335854 (first received 14 April 2011).
Kushida 2011 {published data only}
- Kushida CA, Berry RB, Blau A, Crabtree T, Fietze I, Kryger MH, et al. Positive airway pressure initiation: a randomized controlled trial to assess the impact of therapy mode and titration process on efficacy, adherence, and outcomes. Sleep 2011;34(8):1083‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00636181. Positive pressure treatment of obstructive sleep apnea. clinicaltrials.gov/show/nct00636181 (first received 14 March 2008).
Lang 2018 {published data only}
- Lang KP. CBT group intervention to increase CPAP adherence among veterans with sleep apnea: a pilot investigation. ProQuest LLC, 2018. [Google Scholar]
Lettieri 2009 {published data only}
- Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA, et al. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Annals of Internal Medicine 2009;151(10):696‐702. [DOI] [PubMed] [Google Scholar]
- NCT00612157. Continuous positive airway pressure (CPAP) promotion and prognosis ‐ the army sleep apnea program (ASAP) (CPAPASAP). clinicaltrials.gov/show/nct00612157 (first received 11 February 2008).
Lopez‐Martin 2005 {published data only}
- Lopez‐Martin S, Sanchez‐Munoz G, Gonzalez‐Moro JM, Miguel‐Diez J, Pedraza‐Serrano F, Lucas‐Ramos P. CPAP treatment compliance in patients with obstructive sleep apnea syndrome (OSAS) does it improve when the treatment is inhaled under supervision. American Thoracic Society International Conference; 2005 May 20‐25; San Diego. 2005:C29.
Luyster 2018 {published data only}
- Luyster FS, Aloia MS, Buysse DJ, Dunbar‐Jacob J, Martire LM, Sereika SM, et al. A couples‐oriented intervention for positive airway pressure therapy adherence: a pilot study of obstructive sleep apnea patients and their partners. Behavioural Sleep Medicine 2018;17(5):561‐572. Epub 2018 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01377584. COMMunication and Education for the New CPAP Experience (COMMENCE). clinicaltrials.gov/ct2/show/nct01377584 (first received 20 June 2011).
Marques 2017 {published data only}
- Marques S, Bento AR, Monteiro S, Gralho A, Silva F, Duarte M, et al. The impact of a telemedicine monitoring on positive airway pressure in naive obstructive sleep apnea patients' outcomes: a randomized controlled trial. Sleep Medicine Abstract Supp 2017;40:e83. [Google Scholar]
Marshall 2003 {published data only}
- Marshall MJ, Scammels C, Lowe S. Does proactive intervention influence compliance on continuous positive airway pressure therapy (CPAP)?. Respiratory Care 2003;48(11):1094. [Google Scholar]
Moore 2012 {published data only}
- Moore WR, Olson EJ, Vickers Douglas K, Dierkhising RA, Sikkink VK, Heim‐Penokie PC, et al. Can video based positive airway pressure (PAP) education impact acceptance, self efficacy and adherence to PAP in the management of obstructive sleep apnea?. Sleep 2012;35:A170. [Google Scholar]
Nadeem 2013 {published data only}
- Nadeem R, Rishi MA, Srinivasan L, Copur AS, Naseem J. Effect of visualization of raw graphic polysomnography data by sleep apnea patients on adherence to CPAP therapy. Repiratory Care 2013;58(4):607‐13. [DOI] [PubMed] [Google Scholar]
NCT00310310 {published data only}
- Deering S, Liu L, Zamora T, Hamilton J, Stepnowsky C. CPAP adherence is associated with attentional improvements in a group of primarily male patients with moderate to severe OSA. Journal of Clinical and Sleep Medicine 2017;13(12):1423‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00310310. Effect of self‐management on improving sleep apnea outcomes. clinicaltrials.gov/show/nct00310310 (first received 22 March 2017).
NCT00873977 {published data only}
- NCT00873977. Treatment adherence and outcomes in three modalities of continuous positive airway pressure treatment for obstructive sleep apnea. clinicaltrials.gov/show/nct00873977 (first received 2 April 2009).
NCT00939601 {published data only}
- NCT00939601. Evaluating behavioral treatments to improve adherence to CPAP in people with obstructive sleep apnea (BREATHE). clinicaltrials.gov/ct2/show/NCT00939601 (first received 15 July 2009).
NCT01013207 {published data only}
- NCT01013207. Nexus Compliance Study. clinicaltrials.gov/ct2/show/NCT01013207 (first received 13 November2009).
NCT01102920 {published data only}
- NCT01102920. Life‐style changes in obstructive sleep apnea [Health behaviour modifications in obstructive sleep apnea. Tailored behavioural medicine strategies to promote physical activity and weight loss]. clinicaltrials.gov/ct2/show/NCT01102920 (first received 13 April 2010).
NCT01535586 {unpublished data only}
- NCT01535586. A Rrandomized cross over trial of two treatments for obstructive sleep apnea in veterans with post traumatic stress disorder. clinicaltrials.gov/ct2/show/nct01535586 (first received 17 February 2012).
NCT01538069 {unpublished data only}
- NCT01538069. Heart failure and sleep apnea: exercise training and continuous positive airway pressure [Comparison between exercise training and CPAP treatment for patients with heart failure and sleep apnea.]. clinicaltrials.gov/ct2/show/record/NCT01538069 (first received 23 February 2012).
NCT01569022 {published data only}
- El‐Solh AA, Homish GG, Ditursi G, Lazarus J, Rao N, Adamo D, et al. A randomized crossover trial evaluating continuous positive airway pressure versus mandibular advancement device on health outcomes in veterans with posttraumatic stress disorder. Journal of ClinicalSleep Medicine 2017;13(11):1327‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01569022. A randomized cross over trial of two treatments for sleep apnea in veterans with post‐traumatic stress disorder. clinicaltrials.gov/show/nct01569022 (first received 2 April 2012).
NCT01924507 {unpublished data only}
- NCT01924507. Bedside sleep medicine. clinicaltrials.gov/show/nct01924507 (first recieved 16 August 2013).
NCT01960465 {unpublished data only}
- NCT01960465. Race and CPAP effectiveness (RACE). clinicaltrials.gov/ct2/show/record/NCT01960465 (first received 10 October 2013).
NCT02085720 {unpublished data only}
- NCT02085720. Prevalence of OSAS in Chinese elderly and its CPAP compliance. clinicaltrials.gov/ct2/show/nct02085720 (first received 21 August 2014).
NCT02278094 {unpublished data only}
- NCT02278094. The influences of intervention with home‐based recovery activity in obstructive sleep apnea syndrome. clinicaltrials.gov/show/nct02278094 (first received 29 October 2014).
NCT02331992 {unpublished data only}
- NCT02331992. Positive airway pressure program. clinicaltrials.gov/ct2/show/nct02331992 (first received 6 January 2015).
NCT02339597 {unpublished data only}
- NCT02339597. Therapy adherence of APAP therapy initiation in sleep‐lab versus at home. https://clinicaltrials.gov/ct2/show/NCT02339597 (first received 15 January 2015).
NCT02375321 {published and unpublished data}
- NCT02375321. Quality of life in patients with obstructive sleep apnea: the role of CPAP associated to psychological support (QUOLOSA). clinicaltrials.gov/ct2/show/NCT02375321 (first received 2 March 2015). [NCT02375321]
NCT02459548 {unpublished data only}
- NCT02459548. Continuous positive airway pressure (CPAP) for primary care (CPAP‐SU‐MAP). clinicaltrials.gov/ct2/show/nct02459548 (first received 2 June 2015).
NCT02509247 {unpublished data only}
- Anttalainen U, Melkko S, Hakko S, Laitinen T, Saaresranta T. Telemonitoring of CPAP therapy may save nursing time. Schlaf & Atmung [Sleep & breathing]. 2016; Vol. 20 MISC1 ‐ SLP(4):1209‐15. [DOI] [PubMed]
- NCT02509247. Telemonitoring in CPAP treatment. https://clinicaltrials.gov/ct2/show/NCT02509247 first received 27 July 2015.
NCT02553694 {unpublished data only}
- Guralnick AS, Balachandran JS, Szutenbach S, Adley K, Emami L, Mohammadi M, et al. Educational video to improve CPAP use in patients with obstructive sleep apnoea at risk for poor adherence: a randomised controlled trial. Thorax. United Kingdom BMJ Publishing Group, 2017; Vol. 72 MISC1 ‐ SLP(12):1132‐9. [CRSSTD ‐ 6863267 DBP ‐ 2017/07/02 06:00] [DOI] [PubMed]
- Guralnick AS, Balachandran JS, Szutenbach S, Adley K, Emami L, Mohammadi M, et al. Video education to improve cpap adherence (sleep apnea video education for CPAP or save‐CPAP study): a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine. Netherlands American Thoracic Society, 2017; Vol. 195 MISC1 ‐ SLP(Meeting Abstracts):A6544.
- NCT02553694. Sleep Apnea Video Education for CPAP (SAVE‐CPAP). https://clinicaltrials.gov/ct2/show/record/NCT02553694 first received 17 September 2015.
NCT02553902 {unpublished data only}
- NCT02553902. Economic evaluation of treatment modalities for position dependent obstructive sleep apnea. clinicaltrials.gov/ct2/show/record/NCT02553902 (first received 18 September 2015).
NCT02755831 {unpublished data only}
- NCT02755831. Targeted CPAP therapy for OSA in pregnancy. clinicaltrials.gov/show/nct02755831 (first received 29 April 2016).
NCT02779894 {unpublished data only}
- NCT02779894. Sleep apnoea management by a communication based technology (ICT). clinicaltrials.gov/show/NCT02779894 (first received 23 May 2016).
NCT02953028 {unpublished data only}
- NCT02953028. Obstructive sleep apnea ‐ patient specific factors, success rate and compliance. clinicaltrials.gov/show/nct02953028 (first received 2 November 2016).
NCT03007745 {unpublished data only}
- NCT03007745. Remote sleep apnea management. clinicaltrials.gov/ct2/show/NCT03007745 (first received 2 January 2017).
NCT03202602 {unpublished data only}
- NCT03202602. Benefits of telemedicine in CPAP treatment. clinicaltrials.gov/show/nct03202602 (first received 28 June 2017).
NCT03472612 {unpublished data only}
- NCT03472612. OSA recurrence in CPAP withdrawal. clinicaltrials.gov/show/nct03472612 (first received 21 March 2018).
Palmer 2004 {published data only}
- Palmer S, Selvaraj S, Dunn C, Osman LM, Cairns J, Franklin D, et al. Annual review of patients with sleep apnea/hypopnea syndrome‐‐a pragmatic randomised trial of nurse home visit versus consultant clinic review. Sleep Medicine 2004;5:61‐5. [DOI] [PubMed] [Google Scholar]
Pepin 1996 {unpublished data only}
- Pepin JL, Deschaux C, Krieger J, Cornette A, Rodenstein D, Grillier Lanoir V, et al. A three months effective compliance with nasal CPAP: a European cooperative prospective study in 109 patients. European Respiratory Journal 1996;9(23):464s. [DOI] [PubMed] [Google Scholar]
Pepin 1999 {unpublished data only}
- Pepin JL, Krieger J, Rodenstein D, Cornette A, Sforza E, Delguste P, et al. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Respiratory and Critical Care Medicine 1999;160(4):1124‐9. [DOI] [PubMed] [Google Scholar]
Pepin 2018 {unpublished data only}
- Pepin JL. CALLSAS Study: effect of continuous positive airway pressure treatment for obstructive apnea on phone usage habits (CALLSAS). clinicaltrials.gov/show/NCT03537066 (first received 25 May 2018).
Rodgers 2015 {published data only}
- Rodgers B, Brown LK, Lopez S, Glasser J. Increased engagement and adherence in adults with obstructive sleep apnea. Sleep Abstract Supp 2015;38:A182. [Google Scholar]
Ruhle 1999 {unpublished data only}
- Ruhle KH, Randerath W, Quessada J. Optimising CPAP accommodation time by feedback‐supported training phases in patients with obstructive sleep apnea syndrome. Pneumologie 1999;53:540‐3. [DOI] [PubMed] [Google Scholar]
Sanchez‐de‐la‐Torre 2015 {published data only}
- Sanchez‐de‐la‐Torre M, Nadal N, Cortijo A, Masa JF, Duran‐Cantolla J, Valls J, et al. Role of primary care in the follow‐up of patients with obstructive sleep apnoea undergoing CPAP treatment: a randomised controlled trial. Thorax 2015;70(4):346‐52. [DOI] [PubMed] [Google Scholar]
Sanchez‐Quiroga 2018 {published data only}
- Sanchez‐Quiroga MA, Corral J, Gomez‐de‐Terreros FJ, Carmona‐Bernal C, Asensio‐Cruz MI, Cabello M, et al. Primary care physicians can comprehensively manage sleep apnea patients: a non‐inferiority randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2018;198(5):648‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schiefelbein 2005 {published data only}
- Schiefelbein J. Internet interventions for older persons with obstructive sleep apnea: preparedness and problem‐solving confidence [Dissertation]. University of Kansas. University of Kansas, 2005. [Google Scholar]
Signes‐Costa 2005 {unpublished data only}
- Signes‐Costa J, Chiner E, Andreu AL, Pastor E, Llombart M, Gomez‐Merino E, et al. Patient compliance with CPAP home based diagnosis and review preliminary report. European Respiratory Journal 2017;50(61):A3496. [Google Scholar]
Singhal 2016 {published data only}
- Singhal P, Joshi Y, Singh G, Kulkarni S. Study of factors affecting compliance of continuous positive airway pressure (CPAP) in obstructive sleep apnea‐hypopnea syndrome (OSAHS) Study of factors affecting compliance of continuous positive airway pressure (CPAP) in obstructive sleep apnea‐hypopnea syndrome (OSAHS). European Respiratory Journal 2016;48:PA2362. [Google Scholar]
Tarasiuk 2012 {published data only}
- Tarasiuk A, Reznor G, Greenberg‐Dotan S, Reuveni H. Financial incentive increases CPAP acceptance in patients from low socioeconomic background. PLOS One 2012;7(SLP(3)):e33178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tarraubella 2017 {unpublished data only}
- Tarraubella N, Nadal N, Batlle J, Benitez ID, Cortijo A, Urgeles MC, et al. Management of patients with suspected obstructive sleep apnea (OSA) in primary care (PC) compared to sleep unit (SU): a non‐inferiority randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2017;195:A6563. [Google Scholar]
Tatousek 2015 {published data only}
- Tatousek J, Lacroix J, Visser T, Teuling N. Promoting adherence to CPAP with tailored education and feedback: A randomized controlled clinical trial. Sleep 2015;38:A182. [Google Scholar]
Taylor 2006 {unpublished data only}
- Taylor Y, Eliasson A, Andrada T, Kristo D, Howard R. The role of telemedicine in CPAP compliance for patients with obstructive sleep apnea syndrome. Sleep and Breathing 2006;10(3):132‐8. [DOI] [PubMed] [Google Scholar]
- Taylor YL. A comparison between a telemedicine and traditional management model of care with nasal continuous positive airway pressure use among individuals with obstructive sleep apnea syndrome [Dissertation]. George Washington University, 2003. [Google Scholar]
- Taylor YL, Eliasson A, Kristo D, Andrala T, Bigott T, Ephraim P, et al. Can telemedicine improve compliance with nasal CPAP? [Abstract]. Sleep 2003;26:A263. [Google Scholar]
Wenzel 2008 {published data only}
- Wenzel M, Neifer C, Wenzel G, Kerl J, Jurgens K, Suchi S, et al. Follow‐up examination and probatory CPAP therapy of obstructive sleep apnoea increase the long‐term compliance [Poststationäre nachsorge und probatorische CPAP‐therapie schlafbezogener obstruktiver atemstörungen steigern die langzeitcompliance]. Penumologie 2008;62(2):75‐9. [DOI] [PubMed] [Google Scholar]
Wiese 2005 {published data only}
- Wiese HJ, Boethel C, Phillips B, Peters J, Viggiano T. CPAP compliance: video education may help!. Chest 2002;122(4 suppl):P256. [DOI] [PubMed] [Google Scholar]
- Wiese HJ, Boethel C, Phillips B, Wilson JF, Peters J, Viggiano T. CPAP compliance: Video education may help!. Sleep Medicine 2005;6(2):171‐4. [DOI] [PubMed] [Google Scholar]
Williams 2014 {published and unpublished data}
- Williams NJ, Jean‐Louis G, Brown CD, McFarlane SI, Boutin‐Foster C, Ogedegbe G. Telephone‐delivered behavioral intervention among blacks with sleep apnea and metabolic syndrome: study protocol for a randomized controlled trial. Trials 2014;15:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Alessi 2018 {unpublished data only}
- Alessi C, Martin JL, Fung CH, Dzierzewski JM, Fiorentino L, Stepnowsky C, et al. Randomized controlled trial of behavioral treatment for coexisting insomnia and obstructive sleep apnea: results in middle‐aged versus older veterans. Journal of the American Geriatrics Society 2018;66((Suppl 2)):S128. [Google Scholar]
- Alessi CA, Martin JL, Fung CH, Dzierzewski JM, Fiorentino L, Stepnowsky C, et al. Randomized controlled trial of an integrated behavioral treatment in veterans with obstructive sleep apnea and coexisting insomnia DBP ‐ 201824. Sleep CNO ‐ CN‐01612581. Netherlands Oxford University Press, 2018; Vol. 41 (Suppl 1):A155.
Aloia 2013a {published data only}
- Aloia M, Harrington J, Cartwright A, Goelz K, Edinger JD, Lee‐Chiong T. Personalized video to improve adherence to PAP therapy DBP ‐ EM:201428. Sleep CNO 2013;36:A407‐8. [Google Scholar]
- Aloia M, Harrington J, Cartwright A, Goelz K, Edinger JD, Lee‐Chiong T. Personalized video to improve adherence to PAP therapy. Sleep. 2013; Vol. 36 MISC1 ‐ SLP():A407‐A408.
Becker 2012 {unpublished data only}
- Becker K, Chang NS, Chang JW, Moss B, Daclan AL, Bertone IE, et al. Effectiveness of a respiratory therapist based CPAP follow‐up program. Sleep Abstract Supp 2012;35:A172. [Google Scholar]
ChiCTR‐ONC‐17013132 {unpublished data only}
- ChiCTR‐ONC‐17013132. Clinical phenotypes and precise treatment of adult OSA (obstructive sleep apnea): a multicenter study. chictr.org.cn/showprojen.aspx?proj=21189 (first received 27 October 2017).
Chin 2018 {unpublished data only}
- Chin K, Murase K, Tanizawa K, Takahashi N, Tsuda T, Ohi M, et al. Telemedicine system for optimizing CPAP adherence in Japan: multicenter randomized trial. American Journal of Respiratory and Critical Care Medicine 2018;197:A7450. [Google Scholar]
Daaz‐Cambrilles 2002 {published data only}
- DAaz‐Cambriles T, DAaz‐Atauri MJ, Parez‐Rojo R, Rey L, Valeri‐Busto V, Lapez‐Encuentra A. CPAP treatment for obstructive sleep apnoea syndrome after CPAP training term. European Respiratory Journal 2002;20(Supp 38):102s. [Google Scholar]
Deng 2017 {unpublished data only}
- Deng ZH, Li JR, Hou Q, Chen NN, Cui ZY, Li LZ, et al. Role of sleep apnea monitoring management platform in the treatment of patients with obstructive sleep apnea hypopnea syndrome. Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery 2017;31:1646‐8;1652. [DOI] [PubMed] [Google Scholar]
Duncan 2018 {unpublished data only}
- Duncan J, Hibbert M, Joffe D, Mohammadieh A, Cohen G, Cistulli P, et al. Effectiveness of a pilot patient‐centered sleep study report in the management of obstructive sleep apnoea. Journal of Sleep Research 2018;27(Suppl 2; e05_12766):P006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Georgoulis 2018 {unpublished data only}
- Georgoulis M, Labrou K, Yiannakouris N, Mourati I, Vagiakis E, Kechribari I, et al. Effects of a weight‐loss Mediterranean lifestyle intervention on obstructive sleep apnea: preliminary results of a randomized controlled clinical trial. Journal of Sleep Research 2018;27((Suppl 1)):149‐50. [Google Scholar]
Kataria 2018 {unpublished data only}
- Kataria L, Sundahl C, Skalina L, Shah M, Pfeiffer M, Balish M, et al. Text message reminders and intensive education improves positive airway pressure compliance and cognition in veterans with traumatic brain injury and obstructive sleep apnea: ANNIE pilot study. Neurology 2018;90(Suppl 1):15. [Google Scholar]
Liaw 2018 {unpublished data only}
- Liaw S‐T, Marks G, Buchanan P, Williamson J, Johnson A, Tam M, et al. Long term follow up of obstructive sleep apnoea in primary care‐a pilot study. Journal of Sleep Research 2018;27((Suppl 2)):P130. [Google Scholar]
Marques 2018 {unpublished data only}
- Marques S, Bento A, Gralho A, Duarte M, Mayoralas S, Caneiras C. Adherence in positive airway pressure in naive obstructive sleep apnea patients: the importance of the first month of treatment and the impact of telemedicine monitoring. Journal of Sleep Research CNO ‐ CN‐01647290. Netherlands Blackwell Publishing Ltd, 2018; Vol. 27 (Suppl 1):154.
- Marques S, Bento A, Gralho A, Duarte M, Mayoralas S, Caneiras C. The impact of a telemedicine monitoring on positive airway pressure in naive obstructive sleep apnea patients' outcomes: a randomized controlled trial. Journal of Sleep Research 2018;27((Suppl 1)):154‐5. [Google Scholar]
Murase 2018 {unpublished data only}
- Murase K, Chin K. The approach to establish telemedicine system of CPAP therapy in Japan. Sleep and Biological Rhythms 2018;16(4):471. [Google Scholar]
Naik 2015 {published data only}
- Naik S, Kreinin I, Qin L, Kryger M. Active remote monitoring of CPAP treatment in obstructive sleep apnea may improve adherence in military veterans. Sleep 2015;38:A422. [Google Scholar]
NCT01259440 {unpublished data only}
- NCT01259440. Using telemedicine to improve veteran sleep apnea care. clinicaltrials.gov/ct2/show/nct01259440 (first received 14 December 2010).
NCT01642160 {unpublished data only}
- NCT01642160. Continous positive airway pressure (CPAP) compliance study (ICAN). clinicaltrials.gov/ct2/show/nct01642160 (first received 17 July 2012).
NCT01715194 {unpublished data only}
- NCT01715194. Telemedicine to enhance adherence to CPAP therapy in patients with OSAS. https://clinicaltrials.gov/ct2/show/NCT01715194 first received 26 October 2012.
NCT01785303 {unpublished data only}
- NCT01785303. Multidisciplinary approach to the treatment of insomnia and comorbid sleep apnea. clinicaltrials.gov/ct2/show/NCT01785303 (first received 7 April 2013).
NCT01796769 {unpublished data only}
- NCT01796769. Multidisciplinary and coordinated follow‐up based on a telemonitoring web platform for improving CPAP compliance in low cardiovascular risk sleep apnea patients : OPTISAS 1 Study. clinicaltrials.gov/ct2/show/nct01796769 (first received 22 February 2013). [NCT01796769]
NCT01848509 {unpublished data only}
- NCT01848509. Telemedicine for sleep apnea patients. clinicaltrials.gov/ct2/show/nct01848509 (first received 7 May 2013).
NCT01916655 {unpublished data only}
- NCT01916655. Improving the frequency and quality of sleep apnea care management. clinicaltrials.gov/ct2/show/nct01916655 (first received 5 August 2013).
- Stepnowsky C, Zamora T. Effect of a self‐management intervention on OSA outcomes [Abstract]. Sleep. 2010; Vol. 33:A163.
NCT02056002 {unpublished data only}
- NCT02056002. Peer‐driven intervention for sleep apnea (PCORI). clinicaltrials.gov/ct2/show/nct02056002 (first received 5 February 2014).
NCT02159885 {unpublished data only}
- NCT02159885, Crescenz MJ. Telemedici ne management of veterans with newly diagnosed obstructive sleep apnea (OSA). clinicaltrials.gov/ct2/show/nct02159885 (first received 10 June 2014).
NCT02641496 {published data only}
- NCT02641496. Cognitive behavioral therapy to increase CPAP adherence in veterans with PTSD. clinicaltrials.gov/ct2/show/record/NCT02641496 (first received 29 December 2015).
NCT02657304 {published data only}
- NCT02657304. Effect of early education on the observance of CPAP treatment (CoachSAS). clinicaltrials.gov/show/nct02657304 (first received 15 January 2016).
NCT03116958 {unpublished data only}
- NCT03116958. MyOSA: management and treatment of patients with obstructive sleep apnea syndrome (OSAS) through a telemedicine system. clinicaltrials.gov/show/nct03116958 (first received 17 April 2017).
NCT03243487 {unpublished data only}
- NCT03243487. Therapy adherence management in veterans. clinicaltrials.gov/show/nct03243487 (first received 9 August 2017).
NCT03345524 {unpublished data only}
- NCT03345524. Predictive analytics and peer‐driven intervention for guideline‐based care for sleep apnea. clinicaltrials.gov/show/nct03345524 (first received 17 November 2017).
NCT03446560 {unpublished data only}
- NCT03446560. Cloud based follow up of CPAP treatment. clinicaltrials.gov/show/nct03446560 (first received 27 February 2018).
NCT03536572 {unpublished data only}
- NCT03536572. Self‐management of continuous positive airway pressure settings (ImPRESS). clinicaltrials.gov/show/nct03536572 (first received 24 May 2018).
NCT03792880 {unpublished data only}
- NCT03792880. Global self‐management telematic support for patients with obstructive sleep apnea (TELESAS). clinicaltrials.gov/ct2/show/nct03792880 (first received 4 January 2019). [NCT03792880]
NCT03835702 {unpublished data only}
- NCT03835702. SAMPAP trial ‐impact of a novel sleep apnea management group intervention on positive airway pressure adherence. clinicaltrials.gov/ct2/show/nct03835702 (first received 8 February 2019). [NCT03835702]
Nilius 2012 {unpublished data only}
- Nilius G, Cottin U, Domanski U, Van'THoog T, Franke K‐J, Burian S, et al. Effects of intensive outpatient training on the adherence of CPAP therapy for patients with OSA: A randomised trial. Somnologie 2016;16 MISC1 ‐ SLP(4):251‐6. [Google Scholar]
Ordonez‐Dios 2013 {unpublished data only}
- Ordonez Dios I, Jimenez Romero A, Garcia Amores M, Villar Pastor B, Feu Collado N, et al. Importance of CPAP school. Improving treatment adherence?. Sleep Medicine 2013;14 MISC1 ‐ SLP:e247. [Google Scholar]
Pak 2016 {unpublished data only}
- Pak KJ, Seoane L, Bakker JP, Bertisch S, Pham C, McNaughton N, et al. Effect of mobile health technology on positive airway pressure adherence in patients with sleep apnea. American Journal of Respiratory and Critical Care Medicine 2016;193:A4185. [Google Scholar]
Park 2014 {unpublished data only}
- Park J, Moore W. Internet‐based CPAP adherence via the net (ICAN) trial. Sleep 2014;37:A122. [Google Scholar]
- Park J, Moore W. Internet‐based CPAP adherence via the net (ICAN) trial DBP ‐ EM:201427. Sleep 2014;37:A122. [Google Scholar]
Peach 2003 {unpublished data only}
- Peach RF, Jelic S, Zhong X, Basner RC, Zimmerman BJ. Effect of self‐regulation and self‐monitoring on CPAP adherence in obstructive sleep apnea (OSA) [Abstract]. Sleep 2003;26:A263. [Google Scholar]
Royant‐Parola 2013 {unpublished data only}
- Royant‐Parola S, Hartley S, Violaine L, Lefevre P, Dagneaux S, Escourrou P. Improving CPAP use with telemonitoring and social media. Sleep Medicine 2013;14:e249‐50. [Google Scholar]
Schoch 2017 {unpublished data only}
- Schoch O, Benz G, Baty F, Niedermann J, Brutsche M. Telemetrically triggered interventions in the first month of cpap treatment‐a prospective, randomized controlled intervention trial in patients with a new diagnosis of obstructive sleep apnea syndrome. European Respiratory Journal 2016;48:PA3431. [Google Scholar]
- Schoch OD, Baty F, Benz G, Niedermann J, Brutsche MH. Telemetrically triggered interventions in the first month of CPAP: 6‐months results of a prospective, randomized controlled trial. Respiration 2017;94(1):82‐3. [Google Scholar]
- Schoch OD, Benz G, Baty F, Niedermann J, Brutsche MH. Telemetrically triggered interventions in the first month of CPAP treatment ‐ A prospective, randomized controlled intervention trial in patients with a new diagnosis of obstructive sleep apnea syndrome. Respiration 2016;91:420‐1. [Google Scholar]
- Schoch OD, Benz G, Baty F, Niedermann J, Brutsche MH. Telemetrically triggered interventions in the first month of cpap treatment‐a prospective, randomized controlled intervention trial in patients with a new diagnosis of obstructive sleep apnea syndrome. Kardiovaskulare Medizin CNO ‐ CN‐01914481 2016;Conference: Joint Annual Meeting of the Swiss Society of Cardiology, the Swiss Society of Cardiac and Thoracic Vascular Surgery and the Swiss Society of Pneumology. 2016; Vol. 19 (5 Supplement 26):26S‐27S.
Shaikh 2009 {unpublished data only}
- Shaikh KR, Zaldivar G, Zarrouf F, Sirbu C, Cameron J, Linton J. Weekly phone calls vs. Brief patient education to improve cpap compliance: a randomized controlled trial [Abstract]. Sleep 2009;32:A215. [Google Scholar]
Stepnowsky 2009 {unpublished data only}
- Stepnowsky C, Zamora T. Effect of a self‐management intervention on OSA outcomes [Abstract]. Sleep. 2013; Vol. 33 MISC1 ‐ SLP MISC2 ‐ 6/12.
- Stepnowsky CJ, Zamora T. Effect of a self‐management intervention on CPAP adherence and treatment efficacy [Abstract]. Sleep 2009;32:A225. [Google Scholar]
Stepnowsky 2014a {unpublished data only}
- NCT00682838. Improving obstructive sleep apnea management via wireless telemonitoring. clinicaltrials.gov/show/nct00682838 (first received 22 May 2008).
- Stepnowsky CJ, Agha Z, Barker R, Zamora T, Sarmiento K. Wireless telemonitoring uses less staff time to achieve acceptable CPAP adherence. Sleep 2014;37:A384‐5. [Google Scholar]
Stepnowsky 2014b {unpublished data only}
- Stepnowsky CJ, Zamora T, Barker R, Sarmiento K. Use of clinical video teleconferencing to improve veterans sleep apnea care. Sleep 2014;37:A146. [Google Scholar]
Suarez 2017 {unpublished data only}
- Suarez MC, Garmendia O, Lugo VM, Moraleda A, Farre R, Guerrero G, et al. Simple telemedicine intervention to improve CPAP compliance on OSA patients to minimal (>4 h) and optimal (> 5.5 h) use: study design (CPAP‐rescue). Sleep Medicine 2017;40(1):e317. [Google Scholar]
Tolson 2016 {unpublished data only}
- Tolson J, Miles JC, Bartlett DJ, Barnes M, Jackson ML. An intensive CPAP program to improve treatment adherence and self‐efficacy in patients with obstructive sleep apnea. Sleep 2016;39:A150‐1. [Google Scholar]
Van Der Kleij 2018 {unpublished data only}
- Kleij S, Hanraets B, Backer I, Asin J. The effectiveness of remote monitoring in improving CPAP compliance: a randomised, controlled study. Journal of Sleep Research 2018;27((Suppl 1)):270. [Google Scholar]
Zaldivar 2009 {published data only}
- NCT00945776. Weekly phone calls versus brief patient education to improve continuous positive airway pressure (CPAP) compliance. clinicaltrials.gov/ct2/show/NCT00945776 (first received 24 July 2009).
References to ongoing studies
Abreu 2013 {unpublished data only}
- Abreu T, Silva AM, Canhão C, Paula P, Cristina B. Evaluation of wireless telemonitoring of CPAP therapy in obstructive sleep apnea ‐ TELEPAP study. European Repiratory Journal 2013;42(suppl 57):P2043. [Google Scholar]
Bakker 2017 {unpublished data only}
- Bakker JP, Cailler M, Khan N, Hanson M, Kirkpatrick A, Dudley KA, et al. A randomized trial demonstrating the feasibility of a group‐based peer‐support intervention for maximizing CPAP adherence. American Journal of Respiratry and Critical Care Medicine 2017;95:A6541. [Google Scholar]
- NCT02538419. Group‐based Ppeer support versus individual education for patients undergoing CPAP treatment. clinicaltrials.gov/show/NCT02538419 2 September 2015.
Castronovo 2017 {unpublished data only}
- Castronovo V, Marelli S, Galbiati A, Lombardi GE, Zucconi M, Oldani A, et al. A randomized‐control trial of adherence and acceptance of a telemedicine monitoring system for OSA patients treated with CPAP. Sleep. Conference: 31st anniversary meeting of the associated professional sleep societies, LLC, SLEEP 2017. Netherlands Associated Professional Sleep Societies,LLC, 2017; Vol. 40 MISC1 ‐ SLP():A194‐A195. [CRSSTD ‐ 6863239 DBP ‐ 201723]
- Castronovo V, Marelli S, Galbiati A, Lombardi GE, Zucconi M, Oldani A, et al. Adherence and acceptance of a telemedicine monitoring system for OSA patients treated with CPAP. Sleep 2017;40(suppl_1):A194‐5. [Google Scholar]
Crawford 2016 {published data only}
- Crawford MR, Turner AD, Wyatt JK, Fogg LF, Ong JC. Evaluating the treatment of obstructive sleep apnea comorbid with insomnia disorder using an incomplete factorial design (protocol). Contemporary ClinicalTrials 2016;47:146‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotzian 2018 {published data only}
- Kotzian ST, Schwarzinger A, Haider S, Saletu B, Spatt J, Saletu MT. Home polygraphic recording with telemedicine monitoring for diagnosis and treatment of sleep apnoea in stroke (HOPES Study): study protocol for a single‐blind, randomised controlled trial. BMJ Open 2018;8(1):e018847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seixas 2018 {unpublished data only}
- Seixas AA, Trinh‐Shevrin C, Ravenell J, Ogedegbe G, Zizi F, Jean‐Louis G. Culturally tailored, peer‐based sleep health education and social support to increase obstructive sleep apnea assessment and treatment adherence among a community sample of blacks: study protocol for a randomized controlled trial. Trials 2018;19(1):519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aloia 2007
- Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night‐to‐night variability. Behavioural Sleep Medicine 2007;5(3):229‐40. [DOI] [PubMed] [Google Scholar]
Ancoli‐Israel 2008
- Ancoli‐Israel S, Palmer BW, Cooke JR, Corey‐Bloom J, Fiorentino L, Natarajan L, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer's Disease: a randomized controlled study. Journal of the American GeriatricSociety 2008;56(11):2076‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azarbarzin 2019
- Azarbarzin A, Sands SA, Stone KL, Taranto‐Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease‐related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. European Heart Journal 2019;40(14):1149‐57. [DOI: 10.1093/eurheartj/ehy624] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2016
- Baker CA, Hurley RA, Taber K. Update on obstructive sleep apnea: implications for neuropsychiatry. Journal of Neuropsychiatry and Clinical Neurosciences 2016;28(3):A6‐159. [DOI] [PubMed] [Google Scholar]
Bandura 1982
- Bandura A. Self‐efficacy mechanism in human agency. American Psychologist 1982;37:122‐47. [Google Scholar]
Bandura 1986
- Bandura A. Social Foundations of Thought and Action: a Social Cognitive Theory. Englewood Cliffs, NJ: Prentice‐Hall, 1986. [Google Scholar]
Bandura 2004
- Bandura A. Health promotion by social cognitive means. Health Education and Behavior 2004;31:143‐64. [DOI] [PubMed] [Google Scholar]
Barbé 2012
- Barbé F, Durán‐Cantolla J, Sánchez‐de‐la‐Torre M, Martínez‐Alonso M, Carmona C, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 2012;307(20):2161‐8. [DOI] [PubMed] [Google Scholar]
Beck 1975
- Beck AT. Cognitive Therapy and the Emotional Disorders. ISBN 0‐8236‐0990‐1. Madison, CT: International Universities Press, Inc, 1975. [Google Scholar]
Bollig 2010
- Bollig SM. Encouraging CPAP adherence: it is everyone’s job. Respiratory Care 2010;55(9):1230–6. [PubMed] [Google Scholar]
Bravata 2011
- Bravata DM, Concato J, Fried T, Ranjbar N, Sadarangani T, McClain V, et al. Continuous positive airway pressure: evaluation of a novel therapy for patients with acute ischemic stroke. Sleep 2011;34(9):1271‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bucks 2013
- Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: A meta‐review. Respirology 2013;18(1):61‐70. [DOI] [PubMed] [Google Scholar]
Campos‐Rodriguez 2005
- Campos‐Rodriguez F, Peña‐Griñan N, Reyes‐Nuñez N, Cruz‐Moron I, Perez‐Ronchel J, Vega‐Gallardo F, et al. Mortality in obstructive sleep apnea‐hypopnea patients treated with positive airway pressure. Chest 2005;128(2):624‐33. [DOI] [PubMed] [Google Scholar]
Campos‐Rodriguez 2014
- Campos‐Rodriguez F, Martinez‐Garcia MA, Reyes‐Nuñez N, Caballero‐Martinez I, Catalan‐Serra P, Almeida‐Gonzalez CV. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. American Journal of Respiratory and Critical Care Medicine 2014;189(12):1544‐50. [DOI] [PubMed] [Google Scholar]
Canessa 2011
- Canessa N, Castronovo V, Cappa SF, Aloia MS, Marelli S, Falini A, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. American Journal of Respiratory and Critical Care Medicine 2011;183(10):1419‐26. [DOI] [PubMed] [Google Scholar]
Castronovo 2014
- Castronovo V, Scifo P, Castellano A, Aloia MS, Iadanza A, Marelli S, et al. White matter Integrity in obstructive sleep apnea before and after treatment. Sleep 2014;37(9):1465‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chirinos 2017
- Chirinos DA, Gurubhagavatula I, Broderick P, Chirinos JA, Teff K, Wadden T, et al. Depressive symptoms in patients with obstructive sleep apnea: biological mechanistic pathways. Journal of Behavioral Medicine 2017;40(6):955‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crawford 2014
- Crawford MR, Espie CA, Bartlett DJ, Grunstein RR. Integrating psychology and medicine in CPAP adherence ‐ New concepts?. Sleep Medicine Reviews 2014;18(2):123‐39. [DOI] [PubMed] [Google Scholar]
Dalmases 2014
- Dalmases M, Sole‐Padulles C, Bartres‐Faz D, Nunez L, Embid C, Torres M, et al. Obstructive sleep apnea in elderly patients: neurocognitive function before and after CPAP treatment (Abstract). American Journal of Respiratory and Critical Care Medicine 2014;189 MISC1:A2418. [Google Scholar]
Dalmases 2015
- Dalmases M, olé‐Padullés C, Torres M, Embid C, Nuñez M, Martinez‐Garcia MA, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA a randomized pilot study. Chest 2015;148(5):1214‐23. [DOI] [PubMed] [Google Scholar]
Delhikar 2019
- Delhikar N, Sommers L, Rayner G, Schembri R, Robinson SR, Wilson S, et al. Autobiographical memory from different life stages in individuals with obstructive sleep apnea. Journal of Neuropsychology 2019;25(3):266‐74. [DOI] [PubMed] [Google Scholar]
Devita 2017
- Devita M, Montemurro S, Zangrossi A, Ramponi S, Marvisi M, Villani D, et al. Cognitive and motor reaction times in obstructive sleep apnea syndrome: A study based on computerized measures. Brain and Cognition 2017;117:26‐32. [DOI: 10.1016/j.bandc.2017.07.002] [DOI] [PubMed] [Google Scholar]
Dong 2018
- Dong R, Dong Z, Liu H, Shi F, Du J. Prevalence, risk factors, outcomes, and treatment of obstructive sleep apnea in patients with cerebrovascular disease: a systematic review. Journal of Stroke and Cerebrovascular Disease 2018;27(6):1471‐80. [DOI] [PubMed] [Google Scholar]
Engleman 1994
- Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with the sleep apnoea/hypopnoea syndrome. Thorax 1994;49(3):263‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faheem 2018
- Faheem N, Stevens S. Systematic review and meta‐analysis of randomized control trials examining efficacy of CPAP for prevention of stroke in patients with moderate to severe obstructive sleep apnea. Neurology 2018;90:15 Supplement. [Google Scholar]
Findley 2000
- Findley L, Smith C, Hooper J, Dineen M, Suratt PM. Treatment with nasal CPAP decreases automobile accidents in patients with sleep apnea. American Journal of Respiratory and Critical Care Medicine 2000;161:857‐9. [DOI] [PubMed] [Google Scholar]
Finkel 2009
- Finkel KJ, Searleman AC, Tymkew H, Tanaka CY, Saager L, Safer‐Zadeh E, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Medicine 2009;10(7):753‐8. [DOI] [PubMed] [Google Scholar]
Fleetham 2011
- Fleetham J, Ayas N, Bradley D, Fitzpatrick M, Oliver TK, Morrison D, et al. Canadian Thoracic Society 2011 guideline update: diagnosis and treatment of sleep disordered breathing. Canadian Respiratory Journal 2011;18(1):25‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gagnon 2014
- Gagnon K, Baril AA, Gagnon JF, Fortin M, Décary A, Lafond C, et al. Cognitive impairment in obstructive sleep apnea. Pathologie Biologie 2014;62(5):233‐40. [DOI] [PubMed] [Google Scholar]
Gagnon 2019
- Gagnon K, Baril AA, Montplaisir J, Carrier J, Beaumont L, D'Aragon C, et al. Disconnection between self‐reported and objective cognitive impairment in obstructive sleep apnea. Journal of Clinical Sleep Medicine 2019;15(3):409‐15. [DOI: 10.5664/jcsm.7664] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gami 2005
- Gami AS, Howard DE, Olson EJ, Somers VK. Day‐night pattern of sudden death in obstructive sleep apnea. New England Journal of Medicine 2005;352(12):1206‐14. [DOI] [PubMed] [Google Scholar]
Garbarino 2018
- Garbarino S, Bardwell WA, Guglielmi O, Chiorri C, Magnavita N, Garbarino S, et al. Association of anxiety and depression in obstructive sleep apnea patients : a systematic review and meta‐analysis association of anxiety and depression in obstructive sleep apnea. Behavioral Sleep Medicine 2018;00(00):1‐23. [DOI] [PubMed] [Google Scholar]
Garbarino 2018b
- Garbarino S, Scoditti E, Lanteri P, Conte L, Magnavita N Toraldo DM. Obstructive sleep apnea with or without excessive daytime sleepiness: Clinical and experimental data‐driven phenotyping. Frontiers in Neurology 2018;9:505. [DOI: 10.3389/fneur.2018.00505] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giles 2006
- Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001106.pub3] [DOI] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 17 October 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gurubhagavatula 2017
- Gurubhagavatula I, Sullivan S, Meoli A, Patil S, Olson R, Berneking M, et al. Management of obstructive sleep apnea in commercial motor vehicle operators: recommendations of the aasm sleep and transportation safety awareness task force.. Journal of Clinical Sleep Medicine 2017;13(5):745‐8. [DOI: 10.5664/jcsm.6598] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hack 2000
- Hack M, Davies RJ, Mullins R, Choi SJ, Ramdassingh‐Dow S, Jenkinson C, et al. Randomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoea.. Thorax 2000;55:224‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haentjens 2007
- Haentjens P, Meerhaeghe A, Moscariello A, Weerdt S, PoppeK, Dupont A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta‐analysis of placebo‐controlled randomized trials.. Archives of Internal Medicine 2007;167:757‐64. [DOI] [PubMed] [Google Scholar]
Harsch 2004
- Harsch IA, Schahin SP, Radespiel‐Troger M, Weintz O, Jahreib H, Fuchs FS, et al. CPAP treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. American Journal of Respiratory and Critical Care Medicine 2004;169:156–62. [DOI] [PubMed] [Google Scholar]
Hashmi 2014
- Hashmi AM, Khawaja IS. Awakening to the dangers of obstructive sleep apnea. Current Psychiatry 2014;13(2):59‐63. [Google Scholar]
Haynes 1996
- Haynes RB, McKibbon KA, Kanani R. Systematic review of randomised trials of interventions to assist patients to follow prescriptions for medications. Lancet 1996;348:383‐6. [DOI] [PubMed] [Google Scholar]
Haynes 2002a
- Haynes RB, McDonald H, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD000011] [DOI] [PubMed] [Google Scholar]
Haynes 2002b
- McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions. Scientific review. JAMA 2002;288:2868‐79. [DOI] [PubMed] [Google Scholar]
Haynes 2005
- Haynes RB, Yao X, Degani A, Kripalani S, Garg A, McDonald HP. Interventions to enhance medication adherence. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD000011.pub2] [DOI] [PubMed] [Google Scholar]
Haynes 2008
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence.. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD000011.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (edits). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. CAvailable from handbook.cochrane.org. The Cochrane Collaboration.
Higgins 2019
- Higgins JP, Savović J, Page MJ, Sterne JA, on behalf of the RoB2 Development Group. Revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2). sites.google.com/site/riskofbiastool/welcome/rob‐2‐0‐tool/current‐version‐of‐rob‐2 (accessed prior to 17 October 2019).
Ioannidis 2017
- Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analyses: a large survey. Canadian Medical Association Journal 2017;176(8):1091‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2018
- Jackson ML, Mcevoy RD, Banks S, Barnes M. Neurobehavioral impairment and CPAP treatment response in mild‐moderate obstructive sleep apnea. Journal of Clinical Sleep Medicine 2018;14(1):16‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karimi 2015
- Karimi M, Hedner J, Häbe H, Nerman O, Grote L. Sleep apnea related risk of motor vehicle accidents is reduced by continuous positive airway pressure : Swedish Traffic Accident Registry Data. Sleep 2015;38(3):341‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kennedy 2019
- Kennedy B, Lasserson TJ, Wozniak DR, Smith I. Pressure modification or humidification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003531.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2016
- Kim H, Joo E, Suh S, Kim JH, Kim ST, Hong SB. Effects of long‐term treatment on brain volume in patients with obstructive sleep apnea syndrome. Human Brain Mapping 2016;37(1):395‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kribbs 1993
- Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. American Review of Respiratory Disease 1993;147(4):887‐95. [DOI] [PubMed] [Google Scholar]
Krieger 1992
- Krieger J. Long‐term compliance with nasal continuous positive airway pressure (CPAP) in obstructive sleep apnea patients and non‐apneic snorers. Sleep 1992;15(6):S42‐6. [DOI] [PubMed] [Google Scholar]
Lewis 2004
- Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnoea. Sleep 2004;27(1):134‐8. [DOI] [PubMed] [Google Scholar]
Libman 2017
- Libman E, Bailes S, Fichten CS, Rizzo D, Creti L, Baltzan M, et al. CPAP treatment adherence in women with obstructive sleep apnea. Sleep Disorders 2017;2017:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindberg 2006
- Lindberg E, Berne C, Elmasry A, Hedner J, Janson C. CPAP treatment of a population‐based sample—what are the benefits and the treatment compliance?. Sleep Medicine 2006;7:553‐60. [DOI] [PubMed] [Google Scholar]
Lochhead 2010
- Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, et al. Oxidative stress increases blood‐brain barrier permeability and induces alterations in occluding during hypoxia‐reoxygenation. Journal of Cerebral Blood Flow and Metabolism 2010;30(9):1625‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marin 2005
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365(9464):1046‐53. [DOI] [PubMed] [Google Scholar]
Marin 2012
- Marin M, Forner M, Carrizo Santiago J. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012;307(20):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2008
- Marshall NS, Wong KK, Liu PY, Cullen Stewart RJ, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all‐cause mortality: the Busselton Health Study. Sleep 2008;31(8):1079‐85. [PMC free article] [PubMed] [Google Scholar]
Marshall 2014
- Marshall NS, Wong KK, Cullen Stewart RJ, Knuiman MW, Grunstein RR. Sleep apnea and 20‐year follow‐up for all‐cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. Journal of ClinicalSleep Medicine 2014;10(4):355‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez‐Garcia 2009
- Martínez‐García MA, Soler‐Cataluña JJ, Ejarque‐Martínez L, Soriano Y, Román‐Sánchez P, Illa FB, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5‐year follow‐up study. American Journal of Respiratory and Critical Care Medicine 2009;180(1):36‐41. [DOI] [PubMed] [Google Scholar]
Martinez‐Garcia 2012
- Martínez‐García MA, Campos‐Rodríguez F, Catalán‐Serra P, Soler‐Cataluña JJ, Almeida‐Gonzalez C, Cruz Morón I, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long‐term continuous positive airway pressure treatment: a prospective observational study. American Journal of Respiratory and Critical Care Medicine 2012;186(9):909‐16. [DOI] [PubMed] [Google Scholar]
Mazzotti 2019
- Mazzotti DR, Keenan BT, Lim DC, Gottlieb DJ, Kim J, Pack AI. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. American Journal of Respiratory and Critical Care Medicine 2019;200(4):493‐506. [DOI: 10.1164/rccm.201808-1509OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
McEvoy 2016
- McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. New England Journal of Medicine 2016;375(10):919‐31. [DOI] [PubMed] [Google Scholar]
Mehrtash 2019
- Mehrtash M, Bakker JP, Ayas N. Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea. Lung 2019;197:115‐21. [DOI] [PubMed] [Google Scholar]
Miller 1994
- Miller WR, DiClemente CC, Rychtarik RG, Zweben A. Motivational Enhancement Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence. Vol. 2.. Rockville, MD: U.S. Department of Health and Human Services, 1994. [Google Scholar]
Mokhlesi 2016
- Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. European Respiratory Journal 2016;47:1162‐9. [DOI] [PubMed] [Google Scholar]
Myhill 2012
- Myhill PC, Davis WA, Peters KE, Chubb SA, Hillman D, Davis TM. Effect of continuous positive airway pressure therapy on cardiovascular risk factors in patients with type 2 diabetes and obstructive sleep apnea. Journal of Clinical Endocrinology and Metabolism 2012;97(11):4212‐8. [DOI] [PubMed] [Google Scholar]
Nieuwlaat 2014
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD000011.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohayon 2003
- Ohayon MM. The effects of breathing‐related sleep disorders on mood disturbances in the general population. Journal of Clinical Psychiatry 2003;64(10):1195‐200. [DOI] [PubMed] [Google Scholar]
Olaithe 2013
- Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: a meta‐analysis. Sleep 2013;36(9):1297‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olaithe 2018
- Olaithe M, Bucks RS, Hillman DR, Eastwood PR. Cognitive deficits in obstructive sleep apnea: Insights from a meta‐review and comparison with deficits observed in COPD , insomnia, and sleep deprivation. Sleep Medicine Reviews 2018;38:39‐49. [DOI] [PubMed] [Google Scholar]
Osorio 2015
- Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, et al. Sleep‐disordered breathing advances cognitive decline in the elderly. Neurology 2015;84(19):1964‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pan 2014
- Pan W, Kastin AJ. Can sleep apnea cause Alzheimer's disease?. Neuroscience and Biobehavioral Reviews 2014;47:656‐69. [DOI] [PubMed] [Google Scholar]
Patil 2019
- Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure : an American Academy of Sleep Medicine Clinical Practice Guideline. Journal of Clinical Sleep Medicine 2019;15(2):335‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedrosa 2013
- Pedrosa RP, Drager LF, Paula LK, Amaro AC, Bortolotto LA, Lorenzi‐Filho G. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest 2013;144(5):1487‐94. [DOI: 10.1378/chest.13-0085] [DOI] [PubMed] [Google Scholar]
Pepin 1995
- Pepin L, Leger P, Veale D, Langevin B, Robert D, Levy P. Side effects of nasal continuous positive airway pressure in sleep apnea syndrome: study of 193 patients in two French sleep centers. Chest 1995;107:375‐81. [DOI] [PubMed] [Google Scholar]
Peppard 2000
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep disordered breathing and hypertension. New England Journal of Medicine 2000;342:1378–84. [DOI] [PubMed] [Google Scholar]
Pepperell 2002
- Pepperell JC, Ramdassingh‐Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002;359:204‐10. [DOI] [PubMed] [Google Scholar]
Phillipson 1993
- Phillipson EA. Sleep apnea ‐‐ a major public health problem (Editorial). New England Journal of Medicine 1993;328(17):1271‐3. [DOI] [PubMed] [Google Scholar]
Prochaska 1983
- Prochaska JQ, Diclemente CC. Stages and processes of self‐change of smoking: toward an integrative model of change. Journal of Consulting and Clinical Psychology 1983;51(3):390‐5. [DOI] [PubMed] [Google Scholar]
Punjabi 2009
- Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, et al. Sleep‐disordered breathing and mortality: a prospective cohort study. PLOS Medicine 2009;6(8):e1000132. [DOI: 10.1371/journal.pmed.1000132] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pépin 1999
- Pépin J‐L, Krieger J, Rodenstein D, Cornette A, Sforza E, Delguste P, et al. Effective compliance during the first 3 months of continuous positive airway pressure. American Journal of Respiratory and CriticalCare Medicine 1999;160:1124‐9. [DOI] [PubMed] [Google Scholar]
Reeves‐Hoche 1994
- Reeves‐Hoche MK, Meck R, Zwillich CW. Nasal CPAP: and objective evaluation of patient compliance. American Journal of Respiratory and Critical Care Medicine 1994;149:149‐54. [DOI] [PubMed] [Google Scholar]
Richards 2019
- Richards KC, Gooneratne N, Dicicco B, Hanlon A, Moelter S, Onen F, et al. CPAP adherence may sow 1‐year cognitive decline in older adults with mild cognitive impairment and apnea. Journal of the American Geriatrics Society 2019;67:558‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotenberg 2016
- Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. Journal of Otolaryngology 2016;45(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saunamaki 2007
- Saunamaki T, Jehkonen M. Depression and anxiety in obstructive sleep apnea syndrome: a review. Acta Neurologica Scandinavica 2007;116(5):277‐88. [DOI] [PubMed] [Google Scholar]
Sawyer 2011
- Sawyer AM, Gooneratne N, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Medicine Reviews 2011;15(6):343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. The GRADE Working Group, 2013, 2013. [Google Scholar]
Schwab 2013
- Schwab RJ, Badr SM, Epstein LJ, Gay PC, Gozal D, Kohler M, et al. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems the optimal monitoring strategies and outcome measures in adults. American Journal of Respiratory and Critical Care Medicine 2013;188(5):613‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Senaratna 2016
- Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review.. Sleep Medicine Reviews 2016;34:70‐81. [DOI: 10.1016/j.smrv.2016.07.002] [DOI] [PubMed] [Google Scholar]
Somers 2008
- Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on. Journal of the American College of Cardiology 2008;52(8):686‐717. [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials.. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Stradling 2000
- Stradling JR, Davies RJ. Is more NCPAP better?. Sleep 2000;23(Suppl 4):S150‐3. [PubMed] [Google Scholar]
Stranks 2016
- Stranks EK, Crowe SF. The cognitive effects of obstructive sleep apnea: an updated meta‐analysis. Archives of Clinical Neuropsychology 2016;31:acv087. [DOI] [PubMed] [Google Scholar]
Sullivan 1981
- Sullivan CE, Issa FG, Berthon‐Jones M. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981;1(8225):862–5. [DOI] [PubMed] [Google Scholar]
Sutherland 2018
- Sutherland K, Almeida FR, Chazal P, Cistulli PA. Prediction in obstructive sleep apnoea: diagnosis, comorbidity risk, and treatment outcomes. Expert Review of Respiratory Medicine 2018;12(4):293‐307. [10.1080/17476348.2018.1439743] [DOI] [PubMed] [Google Scholar]
Thunstrom 2016
- Thunstrom E, Manhem K, Rosengren A, Peker Y. Blood pressure response to losartan and continuous positive airway pressure in hypertension and obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine 2016;193(3):310‐20. [DOI] [PubMed] [Google Scholar]
Tregear 2009
- Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta‐analysis. Journal of Clinical Sleep Medicine 2009;5(6):573‐81. [PMC free article] [PubMed] [Google Scholar]
Verstraeten 2004
- Verstraeten E, Cluydts R, Pevernagie D, Hoffmann G. Executive function in sleep apnea: controlling for attentional capacity in assessing executive attention. Sleep 2004;27(4):685‐93. [PubMed] [Google Scholar]
Wang 2017
- Wang Y, Ai Li, Luo J, Li R, Chai Y, He X, et al. Effect of adherence on daytime sleepiness, fatigue, depression and sleep quality in the obstructive sleep apnea/hypopnea syndrome patients undertaking nasal continuous positive airway pressure therapy. Patient Preference and Adherence 2017;11:769‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weaver 2007
- Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007;30(6):711‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weaver 2010
- Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: Implications for future interventions. Indian Journal of Medical Research 2010;131:245‐58. [PMC free article] [PubMed] [Google Scholar]
Yaffe 2011
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. Jama 2011;306(6):613‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yaggi 2005
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. New England Journal of Medicine 2005;353(19):2034‐41. [DOI] [PubMed] [Google Scholar]
Yang 2015
- Yang M, Huang Y, Lan C, Wu Y, Huang K. Beneficial effects of long‐term CPAP treatment on sleep quality and blood pressure in adherent subjects with obstructive sleep apnea. Respiratory Care 2015;60(12):1810‐8. [DOI] [PubMed] [Google Scholar]
Young 2002
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. American Journal of Respiratory and Critical Care Medicine 2002;165(9):1217‐39. [DOI] [PubMed] [Google Scholar]
Young 2002a
- Young T, Shahar E, Nieto J, Redline S, Newman AB, Gottlieb DJ, et al. Predictors of sleep‐disordered breathing in community‐dwelling adults. Archives of Internal Medicine 2002;162:893‐900. [DOI] [PubMed] [Google Scholar]
Young 2008
- Young T, Finn L, Peppard PE, Szklo‐Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen‐year follow‐up of the Wisconsin sleep cohort. Sleep 2008;31(8):1071‐8. [PMC free article] [PubMed] [Google Scholar]
Young 2009
- Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort Study. WMJ: Wisconsin Medical Journal 2009;108(5):246‐9. [PMC free article] [PubMed] [Google Scholar]
Yu 2017
- Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: A systematic review and meta‐analysis. JAMA 2017;318(2):156‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2019
- Zheng D, Xu Y, You S, Hackett ML, Woodman RJ, Li Q, et al. Effects of continuous positive airway pressure on depression and anxiety symptoms in patients with obstructive sleep apnoea: results from the sleep apnoea cardiovascular Endpoint randomised trial and meta‐analysis. EClinicalMedicine 2019;11:89‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimmerman 2006
- Zimmerman ME, Arnedt JT Stanchina M, Millman RP, Aloia MS. Normalization of memory performance and positive airway pressure adherence in memory‐impaired patients with obstructive sleep apnea. Chest 2006;130(6):1772‐8. [DOI] [PubMed] [Google Scholar]
Zinchuk 2017
- Zinchuk AV, Gentry MJ, Concato J, Yaggi HK. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches.. Sleep Medicine Reviews 2017;35:113‐23. [DOI: 10.1016/j.smrv.2016.10.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Haniffa 2004
- Haniffa M, Lasserson TJ, Smith I. Interventions to improve compliance with continuous positive airway pressure for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD003531.pub2] [DOI] [PubMed] [Google Scholar]
Smith 2009b
- Smith I, Nadig V, Lasserson TJ. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines for adults with obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007736] [DOI] [PubMed] [Google Scholar]
Wozniak 2014
- Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD007736.pub2] [DOI] [PubMed] [Google Scholar]


