Figure 9.
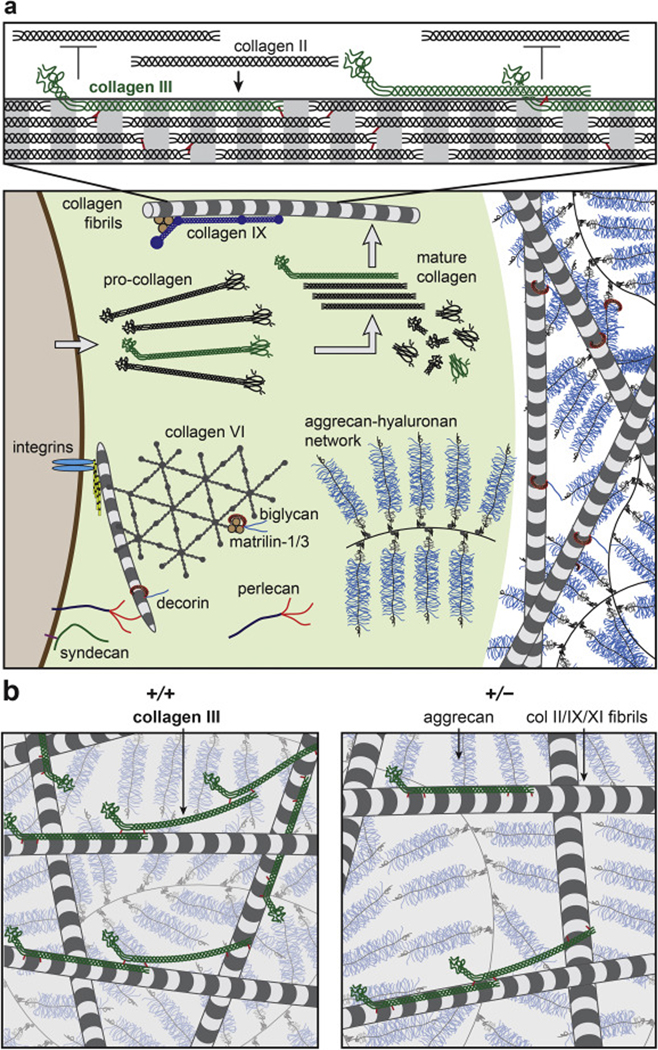
Schematic illustration of the working hypothesis on the structural role of collagen III in articular cartilage ECM, inspired by [19, 40, 62]. a) Upper panel: Collagen III co-assembles with collagen II on fibrillar surfaces during the initial phase of collagen fibrillogenesis in the PCM, and forms covalent cross-links with collagen II and other collagen III molecules. The un-processed N-propeptide limits the lateral growth of collagen II fibrils (collagens IX and XI are not shown to increase clarity). Lower panel: The PCM has distinctive structure and composition in comparison to the further-removed T/IT-ECM, as characterized by the localization of collagen VI, perlecan and biglycan. In the PCM, collagen III could play a role in regulating the initial stage of collagen II fibrillogenesis. b) Reduction of collagen III increases the fibril diameter and heterogeneity in cartilage matrix, and alters the covalent cross-linking patterns of the fibrillar network. This could potentially alter the molecular conformation of aggrecan aggregates. In the schematics, the packing densities of collagen fibrils and aggrecan networks are reduced to increase clarity.
