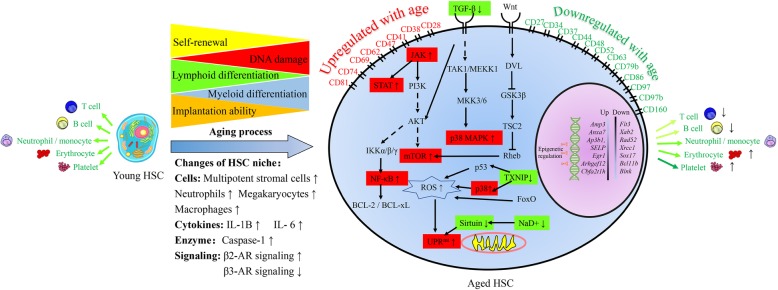
Fig. 1.
Functional alterations and HSC aging mechanisms. Aging negatively affects HSC functions, including decreasing self-renewal ability and myeloid/platelet-biased differentiation and impairing implantation ability. The intrinsic mechanisms are illustrated at the gene level, signaling pathway level, and epigenetic level. HSC aging is accompanied by some cell surface markers being upregulated with age (such as CD28, CD38, CD41, CD47, CD62, CD 69, CD74, and CD81) and some being downregulated with age (such as CD27, CD34, CD37, CD44, CD48, CD52, CD63, CD79b, CD86, CD97, CD97b, and CD160). Furthermore, aged HSCs show different expression levels of specific genes. For example, Amp3, Anxa7, Ap3b1, SELP, Egr1, Arhgef12, and Cbfa2t1h are upregulated, and Flt3, Xab2, Rad52, Xrcc1, Sox17, Bcl11b, and Blnk are downregulated. In addition, some signaling pathways are activated/repressed during HSC aging, including the JAK/STAT-NF-κB-mTOR pathway, TGF-β pathway, Wnt pathway, and ROS and UPRmt pathway. Age-related epigenetic regulation includes DNMT1, DNMT3A, DNMT3B, H3K4me3, and H3K27me3. Extrinsic mechanisms include HSC-surrounding cells (including MSCs, neutrophils, megakaryocytes, and macrophages), cytokines (including IL-6 and IL-1B), enzymes (including caspase-1), and β-adrenergic nerve signaling (including increased β2-AR signaling and decreased β3-AR signaling). The red box indicates that the molecule is upregulated with age, and the green box indicates that the molecule is downregulated with age