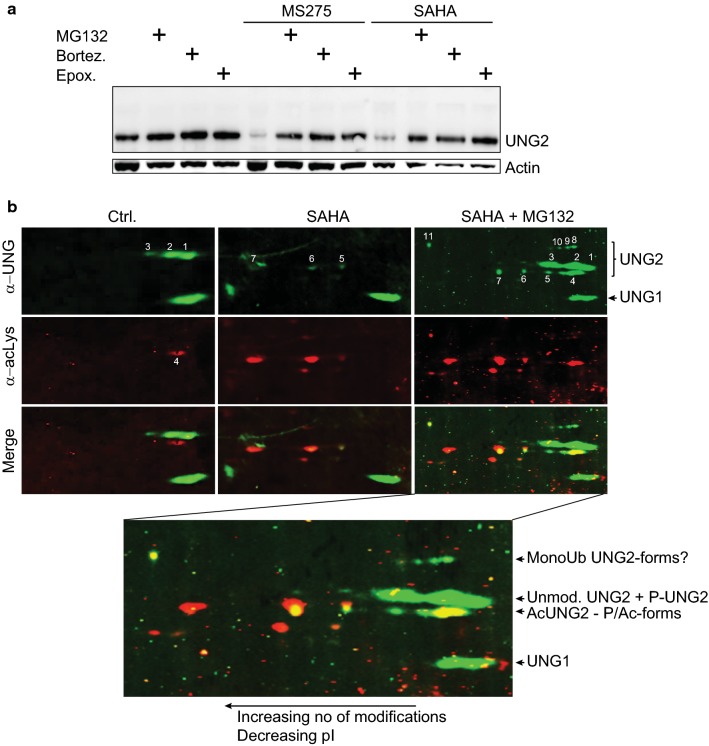
Fig. 5.
a Proteasome inhibitors prevent HDACi-mediated UNG2 depletion. HEK293 cells were treated for 24 h with 5 µM MS-275, 2 µM SAHA or DMSO control in the presence or absence of proteasome inhibitors as indicated (10 µM MG132, 1 µM epoxomicin, 10 µM bortezomib). b 2D-PAGE blots of HEK293 cell extracts after 24 h treatment with DMSO, 2 µM SAHA or 2 µM SAHA + 10 µM MG132 probed with anti-UNG2 PU59 polyclonal antibodies and anti acetyllysine monoclonal antibodies, followed by Alexa fluor 800 (anti-rabbit) or 680 (anti-mouse) secondary antibodies, respectively. Control cells (left panels), showed a pattern of UNG2 isoforms resembling that earlier observed [47], consisting of unmodified, mono- and diphosphorylated UNG2 (designated 1, 2 and 3, respectively). A slightly faster migrating acetylprotein, designated 4 could represent an acetylated isoform as acetylation may induce faster electrophoretic migration and acidic shift in 2D-PAGE, compared to their unmodified forms [84, 85]. The middle panel show cells treated with SAHA (2 µM, 24 h). Strikingly, UNG2 forms 1, 2 and 3 are absent, instead, three novel and faint UNG2 isoforms (designated 5, 6 and 7) are evident, overlapping with acetyllysine signals. Combined SAHA (2 µM) treatment and proteasome inhibition by MG132 (10 µM) (right panels) restored isoforms 1, 2 and 3 and enhanced the acetylated forms 5, 6 and 7. Strikingly, strong UNG2 and acetyl staining was now observed conforming to isoform 4 in the control cells. Several other faint UNG2-positive spots were observed, with varying degree of acetylation signal (bottom enlarged panel). In addition, several isoforms having electrophoretic 2nd dimension migration conforming to monoubiquitinylated UNG2 [47] were observed, of which isoforms 8, 9 and 10 overlapped with weak-, and isoform 11 with strong acetyllysine staining. Notably, isoform 11 conforms well to a multiple phosphorylated and mono-ubiquitinylated isoform of UNG2 observed in G2-phase HeLa [47]. Here we also observe faint signal in S-phase corresponding to isoforms 4, 5, 6, and may indicate a role of acetylation of UNG2 in S-phase (Fig. 4e)