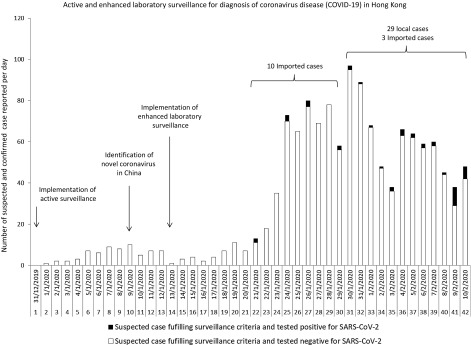
Fig. 1.
Active and enhanced laboratory surveillance for diagnosis of SARS-CoV-2 in Hong Kong. Both calendar date and day after official announcement of a cluster of pneumonia in Wuhan, Hubei Province, by the PRC National Health Commission on December 31, 2019, are shown. From day 1 to day 20, pan-coronavirus PCR with modification to detect 23 coronaviruses known to be present in human, animals, and bats was used. From day 21 onward, real-time PCR targeting the E gene of the SARS-CoV-2/SARS-like coronavirus was performed using the LightMix Modular SARS and Wuhan CoV E-gene mix (TIB Molbiol, Berlin, Germany) and the LightCycler Multiplex RNA Virus Master Kit (Roche Diagnostics, Mannheim, Germany).