Abstract
Attention bias modification (ABM) is a potential intervention in relieving social anxiety symptoms, while its underlying neural mechanisms are not yet understood. The current study included 63 college students with social anxiety. Participants were assigned to the attention modification program (AMP, n = 20), the attention control condition (ACC, n = 20) and the passive waiting group (PW, n = 23). Questionnaires and the emotional Stroop task with EEG recordings were used to assess whether and how the 4-week ABM period affected emotional symptoms and specific emotional processing. Results showed that the two training groups (AMP and ACC) produced comparable emotional improvements and both showed a decrease in negative bias compared with the PW group. The ERP results indicated that despite no significant ERP changes in the PW group, the ACC group exhibited a greater N1, whereas the AMP group exhibited a reduced VPP at the post-test stage compared to the pre-test stage. Besides, both training groups showed a similar late positive potential (LPP) reduction. Notably, the reduction in LPP was positively correlated with behavioral and symptom improvement. Thus, manipulations unique to ABM (face-target contingency) primarily modulate the early attention distribution of material-related stimuli. However, the clinical benefits of attention training may be due to later cognitive-affective mechanisms.
Keywords: Attention bias modification, social anxiety, event-related potentials, interpretation bias, transfer effect
Introduction
Social anxiety disorder (SAD), characterized by the experience of persistent fear in one or more social situations, causes considerable distress and impairs the ability of those affected to function in daily life (APA, 2013). Numerous studies have demonstrated a pronounced selective attention bias to threat in people with social anxiety (Amir et al., 2003). This maladaptive form can manifest as facilitated engagement with threat (Ohman & Mineka, 2001), difficulty to disengage attention away from threat (Arrington et al., 2000; Amir et al., 2003; Derryberry & Reed, 2002; Buckner et al., 2010; Gorlin & Teachman, 2015) or a general reduction in attention control (Eysenck & Derakshan, 2011). All of these mechanisms are affected in social anxiety and contribute to its etiology (Bar-Haim et al., 2007; Cisler & Koster, 2010).
Given the putative pathological association between negative attention bias and social anxiety, attention bias modification (ABM), a systematic training program aimed at altering or correcting exaggerated negative attention biases (Amir et al., 2008), has received widespread interest from researchers and clinicians. In a typical ABM dot-probe task trial, a detection probe always appears in the position that the neutral stimulus previously appeared; thus, individuals implicitly focus more on neutral stimuli than on negative stimuli (Macleod et al., 1986). Over the last decade, researchers have demonstrated the promise of ABM in mitigating social phobia effects, such as reducing levels of anxiety arousal during public speaking, as well as decreasing overall levels of distress (Amir et al., 2008; Li et al., 2008; Amir et al., 2009; Schmidt et al., 2009; Amir et al., 2010; Klumpp & Amir, 2010; Heeren et al., 2011; De Voogd et al., 2014). Meta-analyses have revealed limited but valid clinical effects for ABM in alleviating anxiety symptoms and stress reactivity (Hakamata et al., 2010; Hallion & Ruscio, 2011; Mogoase et al., 2014). The lower costs and ease of access afforded by its administration through a computer make ABM a promising alternative, or at least a complementary approach, to intervention for social anxiety relative to traditional treatments (Bar-Haim, 2010).
However, how ABM training benefits social phobia is still unclear. Heeren et al. (2011) split individuals with social anxiety into groups that were required to either disengage from threat stimuli or engage with non-threat stimuli. Using this method, they found that disengagement from threat reduced behavioral indices of anxiety, while engagement towards non-threats did not have any effects. This suggests that the active components of ABM training stimuli are more closely related to voluntary unbinding from threat, which involves a process of inhibition to preferential responses (negative orientation). In addition, evidences from functional magnetic resonance imaging (f MRI) indicated that clinical benefits of ABM training may be associated with specific changes in brain activity, such as decreased amygdala activation (Britton et al., 2015) and altered resting state functional connectivity (Li et al., 2016). Further, these training benefits may rely on top-down systems, for example Browning et al. (2010) revealed that ABM training can alter the activation of the prefrontal cortex to emotional stimuli.
Nevertheless, some researchers have argued that attention might be best conceptualized and delineated in terms of discrete neurocognitive subprocesses (Cisler & Koster, 2010). To better investigate the specific processes underlying changes in negative attention biases caused by ABM training, neurocognitive approaches of high temporal resolution, such as event-related potential (ERP), can be adopted.
Previous work has shown some preliminary evidence regarding the neurological process of ABM (mostly the dot-probe tasks); however, the results are inconsistent across studies. Some indicated that ABM does not alter automatic negative orientation (Hunkin, 2014; Osinsky et al., 2014) and instead acts mainly on later processing (Eldar & Bar-Haim, 2010); others argued that the early ERPs were impressionable to ABM, and thus, efficient initial allocation of attention to threat may underlie the positive ABM effects (O’Toole & Dennis, 2012; Dennis-Tiwary et al., 2016). These mixed ERP results might be explained by differences in training duration, baseline levels of attention bias and other personality moderator variables employed by the various studies. Indeed, those studies devoted to investigating the mechanisms of ABM tended to adopt training tasks that were completed in 1 day or even in a single session to simplify the operating process; furthermore, few studies truly involved social anxiety groups. This striking gap encouraged us to reassess the complex question of the ABM mechanism, which should be decoupled into at least two phases. First, which aspects of attention processes are influenced by ABM training? Second, which aspects of these altered attention processes bring clinical benefits to socially anxious individuals? To solve this problem, we aimed to investigate the emotional symptoms and emotional neural process changes before and after extensive ABM training. By examining their associated covariation, we may better understand how ABM training affects individuals with social anxiety.
We employed the emotional Stroop paradigm as the index for training transfer effects, that is, to test how ABM training benefits this independent task. The emotional Stroop task has been widely used to investigate emotional information processing for psychological disorders, especially anxiety (Williams et al., 1996). During the task, longer reaction times (RTs) are associated with color-naming of threatening stimuli, whereas neutral stimuli reveal the presence of an attention bias (Askew et al., 2015; Thomas et al., 2007). The emotional Stroop task and dot-probe task (the training task) do not involve identical mental processes, but both represent negative attention bias at the individual level (Mogg et al., 2000). A positive relationship between attention allocation measures from two tasks can be empirically observed (Brosschot et al., 1999). Clinically, Khanna et al. (2015) reported that after dot-probe ABM training, participants with posttraumatic stress disorder no longer exhibited longer color-naming latencies for negative stimuli compared to neutral ones. Further, the extent of attention bias related to social anxiety, as represented by the emotional Stroop task performance, is also sensitive to various interventions, such as exposure and cognitive restructuring (Nortje & Posthumus, 2012).
In our emotional Stroop task, colored emotional faces were employed as the naming targets due to their pronounced effect. Indeed, facial expressions are particularly significant for those with social anxiety as they convey important information concerning self-evaluation and social value (Gilboa-Schechtman et al., 1999). Negative faces such as disgust and fear, presenting low social acceptability and threat, tend to induce greater attention bias and, following social avoidance (Pishyar et al., 2004), thus were frequently used to assess attentional bias in social anxiety (e.g. Amir et al., 2009) and served as the training material of ABM (Bar-Haim, 2010).
EEG signals were simultaneously recorded during the task to examine basic neural changes. Specifically, N1, VPP (vertex positive potential) and LPP (late positive potential) were empirically selected as early and late components to investigate how ABM influences dynamic temporal changes in attentional processing.
N1 reflects feature detection and sensory attention capture based on the salience of the stimulus (Wascher et al., 2009). In addition to being sensitive to the visuospatial nature of the stimulus (Tokudome & Wang, 2012), frontal N1 is also associated with emotional prominence salience (Fields & Kuperberg, 2012; Stevens et al., 2018). Another early area of concern is face-specific VPP (Jeffreys, 1989, 1996). N170, a better-known potential, is considered to be the polarity reversal of VPP (Wheatley et al., 2011). These two components are from the same brain generator and show identical functional properties (Joyce & Rossion, 2005). In the early stages of the facial recognition process, VPP/N170 can be distinguished from ERPs induced by basic physical attributes (Ganis et al., 2012) and are associated with concrete discrimination and selective perception of faces (Batty & Taylor, 2003). In addition to early and automatic stages of information processing (Williams et al., 1996), attention biases underlying emotional Stroop interference are also assumed to operate on cognitive control processes (Amir et al., 1996). LPP, which typically begins ~300 ms after stimulus onset and sustains for more than a second (Hajcak et al., 2006), can be an appropriate indicator of later volitional regulation while facing emotional challenge (Imbir et al., 2017).
In the current study, we evaluated the effect of 4 weeks of multisession ABM training with self-reported emotional states, laboratory tasks (the emotional Stroop task) and EEG recording as an independent measure of attention bias and emotional processing. According to previous studies (Hakamata et al., 2010), we hypothesized that the social anxiety symptoms would be alleviated during training; the attention bias, as reflected by the emotional Stroop task, would decrease as a consequence of the ABM procedure (Khanna et al., 2015). We also expected to observe the training transfer of ABM on the emotional Stroop task referring to ERPs. Since previous literature has not agreed on which stage of ERP components were more sensitive to ABM training, we hypothesize that ABM may alter both early and late ERPs (N1, VPP and LPP) amplitudes in facial emotional Stroop tasks. Additionally, given to the evidence that clinical benefit of ABM as a possible consequence of cognitive control (Heeren et al., 2011), we predict that individual improvements in emotional states will be more closely associated with late component changes indexed by LPP.
Method
Participants
Sixty-three college students with social anxiety from a sample pool of 1328 participants of Asian Chinese students were selected in our study. All participants met the following inclusion criteria: (a) score above 32 on either the fear or avoidance subscale of the Liebowitz Social Anxiety Scale self-report version (LSAS-SR; Baker et al., 2002) and total scores of LSAS-SR equal or greater to 60; (b) score within the top 20% on the Social Interaction Anxiety Scale (SIAS; Mattick & Clarke, 1998); (c) no other psychological treatments undertaken during training; (d) no antipsychotic medications were taken; and (e) no other diagnosed mental disorders.
The participants were assigned to the attention modification program (AMP) group (n = 20, years = 20.05 ± 1.00, males = 2), the attention control condition (ACC) group (n = 20, years = 19.94 ± 1.03, males = 3) and the passive waiting (PW) group (n = 23, years = 19.96 ± 1.02, males = 4). There was no significant difference in age (F(2, 60) = 0.44, P = 0.957), gender ratio (χ2 = 3.92, P = 0.864) and screening LSAS scores among three groups (AMP: 67.30 ± 8.43; ACC: 70.75 ± 8.69; PW: 69.56 ± 8.00, F(2, 60) = 0.885, P = 0.418).
The research was approved by the ethics committee of the Institute of Psychology at the Chinese Academy of Sciences and was carried out in accordance with the approved guidelines. All participants provided written informed consent before the formal experiment and received a small payment as compensation.
Procedure
At the pre-test assessment stage, participants in three groups signed informed consent and completed five self-report scales: fear subscale of the LSAS-SR questionnaire, the Brief Fear of Negative Evaluation Scale (BFNE; Leary, 1983), the Social Phobia Scale (SPS; Mattick & Clarke, 1998) and Beck Depression Inventory-II (BDI-II; Beck et al., 1996). More details for the scale measures are provided in the Supplementary data. Next, all participants performed an emotional Stroop task in the electromagnetic shielding chamber so that ERP data could be collected.
One or 2 days after pre-test assessments, participants in two training groups (AMP and ACC) returned to our lab to perform their training task. Training occurred twice a week over a duration of 4 weeks. Each training session in either group lasted ~21 min. The AMP or ACC condition allocation was decided by random computer draw.
One or 2 days after the final training session, all participants including the AMP, ACC and PW groups returned to complete their post-test assessments, which were identical to those used for the pre-test assessments.
ABM training
The ABM procedure in our research consisted of eight sessions of training in total. In each training session, participants performed a dot-probe task identical to that employed by Amir et al. (2008). Facial pictures were selected from the Chinese Facial Affective Picture System (CFAPS; Gong et al., 2011) and the Asian faces of Matsumoto and Ekman’s Japanese and Caucasian Facial Expressions of Emotion (JACFEE; Biehl et al., 1997) to create neutral-neutral and disgust-neutral pairs. A fixation cross was presented at the center of the screen for 500 ms followed by a pair of faces presented at the top and bottom of the screen for 800 ms. After the presentation of faces, a probe (either the letter ‘E’ or ‘F’) appeared in the location of one of the two previously presented faces. Participants were instructed to judge whether the probe was the letter ‘E’ or ‘F’ as quickly and accurately as possible. Each session comprised 160 trials that were repeated three times for a total of 480 trials consisting of all combinations of variables (probe type, E/F; probe position, top/bottom; face type, neutral/disgust; and person, four male faces/four female faces). Participants were able to rest after every 40 trials. In the AMP training, the probe always replaced the neutral faces in trials with disgust-neural face pairs. In ACC training, the probe replaced the disgust faces and neutral faces with equal frequency.
Notably, before formal training manipulations, there was a practice session in each group. This session was a standard dot-probe task despite limited trials (32 trials with 16 probes after neutral faces and 16 probes after disgust faces). It was designed to familiarize the individual with the keystroke response, but it was also analyzed to examine the dynamic change of attention bias. Relevant analyses are available in the Supplementary data.
Emotional Stroop task
We used a modified emotional face-color Stroop task (Lee et al., 2009) accompanied by EEG recording to assess emotional attention bias processing. Twelve disgust, 12 fear and 12 neutral face pictures were selected from the CFAPS, and each valence comprised six male and six female faces of the same size (260*300 pixels). Faces used in this task were different from those used during ABM training and were tinted. Participants were asked to ignore the expression of the faces and instead identify the color that they were tinted (red, yellow, green or blue) by pressing a key (‘D’, ‘F’, ‘J’ or ‘K’ with the middle finger of their left hand, index finger of their left hand, index finger of their right hand or middle finger of their right hand, respectively) as quickly as possible on a keyboard. Face stimuli were presented after a 1500 ms fixation point and for a 300 ms duration in a pseudorandom fashion to ensure that there were no three consecutive appearances of the same color. The duration of interstimulus interval varied randomly between 1700 ± 2000 ms. One hundred and forty-four facial combinations (12 persons*3 expressions*4 colors) were randomly repeated two times, and 288 trials in total were broken down into six blocks. Prior to formal EEG recording, participants were required to perform 20 practice trials to familiarize with the keystroke requirements. Faces in the practice exercise were different from those in the formal experiment. The task was compiled and presented using E-prme2.0.
EEG recording and offline processing
We recorded EEG data during performance of the emotional face-color task for both pre-training and post-training assessment. Electroencephalography (EEG) was continuously recorded with a Neuroscan Aynamp1 Amplifier using a 32 Ag-AgCl electrode cap (Neuroscan Inc., Herndon, VA, USA) placed on the scalp according to the extended International 10/20 system. The data were processed using EEGLAB (Delorme & Makeig, 2004), and epochs were extracted using a window analysis time of 1000 ms (200 ms pre-stimulus and 800 ms post-stimulus) and baseline corrected using the pre-stimulus interval. Signals were averaged across trials and time-locked to the onset of the compound stimuli separately for disgust, fear and neutral in the emotional face-color Stroop task. More details are provided in the Supplementary data.
Data analysis
In general, Group (AMP/ACC/PW) * Time (pre-test/post-test) ANOVAs were applied to the self-report questionnaire scores and behavioral negative bias scores in emotion Stroop task. The incorrect trials (<5%) and the trials with reaction times (RTs) of less than 100 milliseconds and more than 2 s were excluded from further analysis. The attention bias scores were obtained by subtracting the RTs produced by responding to neutral faces from that produced by negative faces. Specifically, the Disgust Bias score (RTs of disgust face minus that of neutral face) and Fear Bias score (RTs of fear face minus that of neutral face) were calculated.
Group (AMP/ACC/PW) * Time (pre-test/post-test) * Face expression (disgust/fear/neutral) were applied to ERP indices. With regards to the distribution of topographic maps, electrodes with the most prominent ERP components were included. Specifically, FZ, FCZ and CZ for the front N1 (usually most prominent in the frontal-parietal area, e.g. Ma et al., 2016); FCZ, CZ and CPZ for the VPP (usually most prominent in the parietal area, e.g. Jeffreys, 1996); and P3, PZ and P4 for the LPP (usually most prominent in the posterior of the brain, e.g. Hajcak et al., 2010) were selected and averaged respectively to reduce type I error. Time windows of N1, VPP and LPP components were established based on average potentials of each task condition. For the final statistical analyses of mean amplitude, the interval of N1 was 95–110 ms, the interval of VPP was 140–180 ms and the interval of LPP was 300–600 ms.
Finally, for the variables that modulated by the AMB intervention, correlation analyses were performed for each group to investigate relationships between macroscopic behavioral changes and their neural underpinnings.
The Greenhouse–Geisser correction was used to compensate for sphericity violations. Bonferroni adjustment was applied for post hoc testing of main effects. Partial eta-squared was reported as an indicator of the effect size in analysis of variance (ANOVA) tests. All these statistical analyses were conducted with SPSS Version 25.0 software.
Results
Questionnaires
We found a significant main effect of time [F(1, 60) = 14.81, P < 0.001, ηp2 = 0.198] and a significant interaction between group and time on the fear subscale of the LSAS-SR [F(2, 60) = 5.34, P = 0.007, ηp2 = 0.151]. Paired t-test (pre- and post- comparisons for each group) indicated that both the ACC and the AMP groups showed a reduction in LSAS-SR (fear) [ACC pre-post: t(19) = 4.88, P < 0.001; AMP pre-post: t(19) = 2.50, P = 0.022], however, the reduction was not observed in the PW group [PW pre-post: t(22) = −0.28, P = 0.784].
We found a significant main effect of time [F(1, 60) = 6.24, P = 0.015, ηp2 = 0.094] and a significant interaction between group and time on BFNE [F(2, 60) = 5.82, P = 0.015, ηp2 = 0.094]. Paired t-test indicated that both the ACC and the AMP groups showed a reduction in BFNE [ACC pre-post: t(19) = 3.11, P = 0.06; AMP pre-post: t(19) = 2.01, P = 0.050]; however, the reduction was not observed in the PW group [PW pre-post: t(22) = −1.25, P = 0.225].
We found a significant interaction between group and time on SPS [F(2, 60) = 3.13, P = 0.050, ηp2 = 0.095]. Paired t-test indicated that the AMP group showed a reduction in SPS [AMP pre-post: t(19) = 2.30, P = 0.033]; the reduction was not observed in the ACC and PW group [ACC pre-post: t(19) = 0.70, P = 0.491; PW pre-post: t(22) = −1.06, P = 0.300].
We found a significant main of time on BDI [F(1, 60) = 4.87, P = 0.031, ηp2 = 0.075]; the BDI scores of post-tests were significantly lower than those of pretests. While the interaction between group and time was nonsignificant [F(1, 60) = 2.34, P = 1.105, ηp2 = 0.072].
The descriptive statistics of questionnaires of three groups are shown in Table 1.
Table 1.
Descriptive statistics of symptom assessments
| AMP (n = 20) | ACC (n = 20) | PW (n = 23) | ||
|---|---|---|---|---|
| LSAS fear | Pre test | 35.30 (10.50) | 40.75 (8.69) | 39.56 (7.95) |
| Post test | 29.60 (12.31) | 32.30 (10.84) | 40.13 (8.57) | |
| BFNE | Pre test | 49.15 (6.22) | 48.95 (5.07) | 49.21 (7.00) |
| Post test | 48.30 (8.11) | 45.55 (5.69) | 50.30 (7.09) | |
| SPS | Pre test | 51.80 (13.07) | 51.10 (10.56) | 53.53 (9.93) |
| Post test | 45.25 (13.73) | 49.30 (12.46) | 56.00 (11.83) | |
| BDI | Pre test | 16.50 (9.90) | 18.40 (7.84) | 17.43 (10.30) |
| Post test | 14.80 (8.11) | 14.20 (6.38) | 17.69 (8.51) | |
AMP: Attention Modification Program; ACC: Attention Control Condition; PW: Passive Waiting group
Behavioral data
To test whether there was negative attention bias in all three groups, we conducted the single-sample t-test (compare to zero.) for the Disgust bias (RTs of disgust faces minus RTs of neutral faces) and the fear bias (RTs of fear faces minus RTs of neutral faces). Results showed that for all the groups, both the Disgust bias and Fear bias scores were significantly higher than zero (Ps < 0.05). In addition, there were no significant differences in negative bias scores among the three groups at the pre-test period (Fs < 1); see Supplementary data.
The two-way ANOVAs of Group (AMP/ACC/PW) * Time (pre-test/post-test) on Disgust Bias revealed a significant main effect of time [F(1, 60) = 10.55, P = 0.002, ηp2 = 0.149], and a significant interaction between time and group [F(2, 60) = 4.06, P = 0.022, ηp2 = 0.119]; paired t-test (pre- and post-comparisons for each group) indicated that both the ACC and the AMP groups showed a reduction of Disgust Bias[ACC: t(19) = 2.92, P = 0.033; AMP: t(19) = 3.19, P = 0.005]; however, the reduction was not observed in the PW group [PW: t(22) = −0.339, P = 0.738]. See Figure 1A.
Fig. 1.
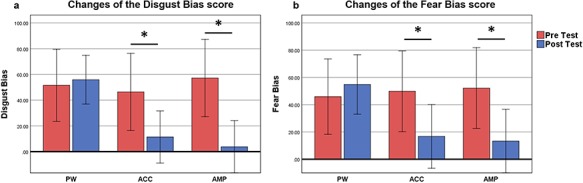
Changes in attention bias score as represented by emotional Stroop task performance. (a) Disgust bias score changes in PW, ACC and AMP, calculated as the difference in RTs in response to disgust faces and neutral faces. (b) Fear bias score change in PW, ACC and AMP, calculated as the difference in RTs in response to fear faces and neutral faces. *P < 0.05. The error bar represents 95% confidence interval.
For the Fear Bias, we also found a significant main effect of time [F(1, 60) = 5.54, P = 0.022, ηp2 = 0.085] and a marginal significant interaction between time and group [F(2, 60) = 2.85, P = 0.058, ηp2 = 0.90]; paired t-test (pre- and post-comparisons for each group) indicated that both the ACC and the AMP groups showed a reduction of Fear Bias[ACC: t(19) = 2.05, P = 0.050; AMP: t(19) = 2.36, P = 0.028]; however, the reduction was not observed in the PW group [PW: t(22) = −0.64, P = 0.531]. See Figure 1B.
The descriptive statistics of emotion Stroop task are shown in Table 2.
Table 2.
Descriptive statistics for reaction times (ms) in the emotion Stroop tasks
| PW (n = 23) | AMP (n = 20) | ACC (n = 20) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||
| PreDisgut | 641.94 | 117.21 | 648.53 | 85.40 | 651.88 | 147.65 | |||
| PreFear | 636.35 | 114.27 | 643.50 | 84.99 | 655.33 | 155.28 | |||
| PreNeutral | 590.41 | 118.39 | 591.33 | 75.74 | 605.47 | 119.25 | |||
| PostDisgust | 636.94 | 117.21 | 599.96 | 79.05 | 620.23 | 112.18 | |||
| PostFear | 635.91 | 112.92 | 609.54 | 84.33 | 625.60 | 126.22 | |||
| PosrNeutral | 581.03 | 106.04 | 596.18 | 92.81 | 608.84 | 94.99 | |||
ERP data
N1
There was a significant interaction between group and time [F(2, 60) = 4.53, P = 0.015, ηp2 = 0.131]. For the ACC group, N1 negativity of facial stimuli at post-training was significantly greater than that at pre-training (P = 0.007). However, for the AMP and PW groups, N1 negativity did not change between the pre-test and post-test period (AMP: P = 0.251; PW: P = 0.276). See Figure 2A.
Fig. 2.
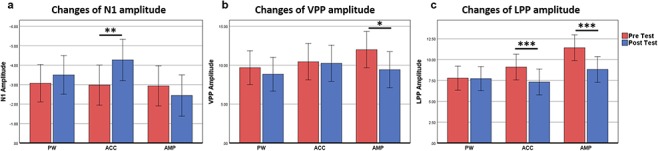
Interaction diagram of time by group of ERPs in the emotional Stroop task. (a) N1 (95–110 ms) at pre- and post-test for PW, AMP and ACC; data shown here is the average amplitude of FZ, FCZ and CZ. (b) VPP (140–180 ms) at pre- and post-test for PW, AMP and ACC; data shown here is the average amplitude of FZ, FCZ and CZ. (c) LPP (300–600 ms) at pre- and post-test for PW, AMP and ACC; data shown here is the average amplitude of P3, PZ and P4. All the facial expressions were merged. *P < 0.05, **P < 0.01, ***P < 0.001 The error bar represents 95% confidence interval.
VPP
There was a significant main effect of facial expression [F(2, 60) = 5.43, P = 0.006, ηp2 = 0.148], the VPP of fear faces was higher than that of neutral faces (P = 0.002). There was a significant main effect of time [F(1, 60) = 10.54, P = 0.002, ηp2 = 0.150] and a significant interaction between group and time [F(2, 60) = 3.50, P = 0.037, ηp2 = 0.104]. In the AMP group, the VPP at post-test was significantly smaller than for pre-test (P = 0.012). However, for the ACC and PW groups, the VPP of facial stimuli did not change significantly between the pre-test and post-test period (ACC: P = 0.702; PW: P = 0.156). See Figure 2B.
LPP
There was a significant main effect of facial expression [F(2, 60) = 13.33, P < 0.001, ηp2 = 0.182]; the LPP of the fear face was higher than disgust (P < 0.001) and neutral faces (P = 0.031). There was a significant main effect of time [F(1, 60) = 40.28, P < 0.001, ηp2 = 0.402] and a significant interaction between group and time [F(2, 60) = 10.87, P < 0.001, ηp2 = 0.266]. In both AMP and ACC groups, the LPP of facial stimuli at post-training was significantly smaller than for pre-training (AMP: P < 0.001; ACC: P = 0.001). However, for the PW group, LPP did not change between the pre-test and post-test periods (P = 0.773). See Figure 2C.
No other significant main or interactive effects were found. The average amplitude and the topography of N1 and VPP at FZ are shown in Figure 3, and LPP at PZ are shown in Figure 4. The statistics and graphics of ERPs on all conditions (group * time * face) are available in the Supplementary data.
Fig. 3.
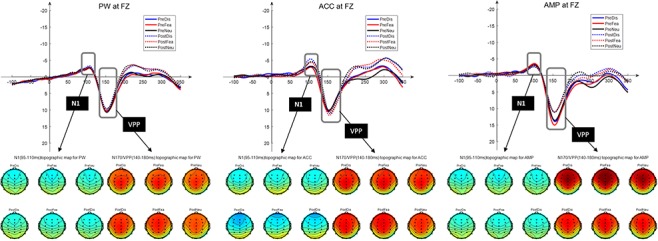
Grand average of early components (N1 and VPP) and topography changes under all conditions at FZ for the PW, ACC and AMP. PreDis: neuronal responses to disgust faces at the pre- test stage; PostDis: neuronal responses to disgust faces at the post- test stage; PreFea: neuronal responses to fear faces at the pre- test stage; PostFea: neuronal responses to fear faces at the post- test stage; PreNeu: neuronal responses to neutral faces at the pre- test stage; PostNeu: neuronal responses to neutral faces at the post- test stage.
Fig. 4.
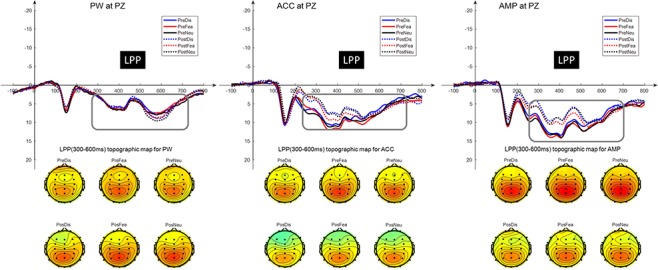
Grand averages of LPP (later component) and topography changes under all conditions at PZ for PW, ACC and AMP.
Correlation between ERPs and the behavioral data
The correlation analysis of ERPs and behavior data revealed that the changes in later but not early components were related to changes in attention bias in behavior and symptoms of social anxiety only for the ACC and AMP groups.
Specifically, alterations in Disgust Bias were positively correlated with changes in LPP amplitude to disgust faces [ACC: r = 0.560, P = 0.010; AMP: r = 0.505, P = 0.023, Both: r = 0.540, P = 0.001] (see Figure 5A), and the reduction of Fear Bias was positively associated with corresponding changes in LPP [ACC: r = 0.442, P = 0.050; AMP: r = 0.481, P = 0.032, both: r = 0.46, P = 0.003] (see Figure 5B). Additionally, changes in induced LPP on neutral faces were positively correlated with changes in self-reported emotional states as reflected by the LSAS (fear) [ACC: r = 0.60, P = 0.005; AMP: r = 0.50, P = 0.023, both: r = 0.48, P = 0.002] (see Figure 5C).
Fig. 5.
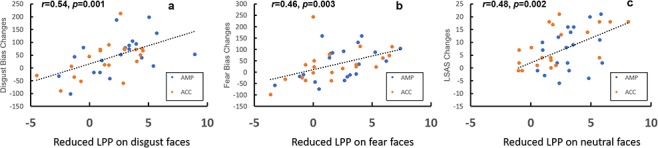
Correlations between reduced LPP, decreased attention bias and social anxiety symptoms. A Reductions in Disgust Bias were positively related to corresponding declines in LPP. B Reductions in Fear Bias were positively related to corresponding declines in LPP. C Declines in LPP in response to neutral faces can predict reductions in social anxiety symptom as indexed by LSAS score. These correlations were significant only in the ACC and AMP groups. The blue and orange dots represent AMP and ACC, respectively. The r value in the figure is the result after combining the two groups.
A correlation matrix for all of these indicators is shown in Supplementary Tables S3 and S4.
Discussion
The aim of this study was to verify the transfer effect of multisession ABM training on social anxiety. Self-report measures and an independent laboratory task with EEG recording were employed to examine the functional mechanism of ABM.
At the behavioral level, we observed that both the ACC and AMP groups exhibited similar improvements in emotional attention control and social anxiety symptoms compared with the waiting group. ERP results, however, showed a separation between early and later ERP component changes within the two training conditions. Specifically, the ACC group showed a greater N1 negativity at the post-training stage compared to the initial pre-test stage. For the subsequent face processing component VPP, the amplitude of facial stimuli at the post-test session significantly decreased in the AMP group, indicating that a face-target contingency within training modulates attentional processing in its early stages. For the late component LPP, both the AMP and ACC groups showed significant declines of amplitude in the post-test period which was not observed in the waiting group. Notably, this LPP reduction was associated with a decrease in behavioral attention bias scores and social anxiety symptoms. This suggests that, although ABM training may allow people to implicitly modulate early hyperactive processing of emotional stimuli, the clinical effects of attention training may primarily come from improvements in general cognitive regulation.
ABM was initially proposed to change the attention bias of individuals in an automatic and implicit way (Bar-Haim, 2010), as reflected by previous studies demonstrating that early attentional stages can be sensitive to ABM training (Dennis & O'Toole, 2014). By using an alternative attentional task, our results also demonstrate an interactive effect between group condition and time on N1 and VPP, indicating that the face-target contingency in training may indeed affect early sensory detection and attentional discrimination.
ERP components in the latent period of 50–100 ms are mainly thought to impact stimulus sensory gating and are related to bottom-up attention capturing determined by stimulus salience (Wascher et al., 2009). Further, the frontal N1 can be sensitive to the affective value of the stimuli (Fields & Kuperberg, 2012; Stevens et al., 2018). In our studies, the ACC group showed increased negativity of N1 at the post-test stage. The erratic face-probe layout in ACC conditions, where the face was merely distraction rather than instruction may elevate the implicit negative attitude to facial stimuli (Schmack et al., 2016), thus possibly accounts for the visual hypersensitivity as indexed by enhanced N1 in the independent task.
VPP, the ERP component of face-specific processing, is independent from earlier sensory gating (Itier & Taylor, 2004) but is also considered to involve noncontrolled automatic processing (Zhu et al., 2010). Our study found that the AMP group experienced decreased VPP amplitude during performance of the emotional Stroop task at the post-training stage, suggesting an improvement in face recognition efficiency (Joyce & Rossion, 2005). As the position of detection stimuli was always fixed at the previous location of neutral faces in the AMP condition, individuals were prompted to obtain a preference for neutral stimuli. However, this manipulation makes disgust faces, on the other hand, a reverse cue for probes. Thus, the group may improve in their general efficiency of face identification. Systematic layout, though unrealized, may implicitly enable individuals in AMP to cultivate a certain sense of dominance or feelings of mastering the face stimulation processing (Klumpp & Amir, 2010), which may also promote overall face processing.
In general, face-probe contingency related to ABM can induce an observable effect on early attention allocation. However, for people with social anxiety, is the clinical benefit of ABM truly due to this early processing efficiency? The answer may be negative. We found that the changes of AMP and ACC in late ERP followed the same pattern, and the LPP amplitude of both groups was significantly reduced compared with the passive waiting group. Furthermore, changes in LPP, rather than in the earlier components of N1 or VPP, were positively associated with changes in behavioral bias scores and emotional self-rating. These results indicate that changes in general cognitive regulation, as indexed by LPP, may in fact be the active principle by which attention training produces clinical effects.
The Stroop task in our research contains a regulatory procedure in which participants are required to disengage from the distractive nature of face stimuli and instead focus on the assignment of color naming. LPP is a classic component of controlled attention to emotion (Hajcak et al., 2009), and its decline in an active task represents a success in voluntary emotional adjustment (Thiruchselvam et al., 2011). In our task, reduced LPP may not represent a simple adaptation to physical simulation, but instead an improvement in the control of emotional conflict. Logically, an observed decrease of LPP in both the AMP and ACC groups may indicate that both conditions lead to improvements in efficiency of individual cognitive-affective regulation. Indeed, this effect is consistent with notions regarding the primary mechanism of ABM training, specifically that it works on the basis of top-down cognitive control and affective regulation (Heeren et al., 2011).
However, unlike previous studies, which only found a decrease in P3 for the AMP group (Eldar & Bar-Haim, 2010), we found that the ACC group also produced a similar late attention-control neural pattern. How might such an effect come about? One possible explanation is that both the AMP and ACC groups provide a way to cultivate attention control, and prolonged training enables all participants to actively or passively improve inhibition and regulation of facial distraction. Indeed, all participants in the analysis declared that either no relationship was clearly detected between faces and the target stimuli. Thus, the comparable late neurological changes as indexed by LPP in both the ACC and AMP groups may be attributed to the common challenges to attention that exist across conditions.
This finding is consistent with a recent fMRI study, in which the AMP and placebo group exhibited different patterns of activation with training; additionally, associations between left amygdala activation and reductions in symptoms were observed regardless of the training group (Britton et al., 2015). Indeed, there is emerging research that suggests basic—rather than bias-specific—training, such as working memory training, brings emotional benefits to individuals (Schweizer et al., 2013). These methods focused on the exercise of basic executive function rather than on a carefully choreographed attention guide. Long-term application of dot-probe tasks in our study, where demands on inhibiting control were required (Amir et al., 2003), may serve as an effective means of training affective control.
Using the facial Stroop task, some findings related to facial expression were observed. A main effect of facial expression was present in our study in which fear faces induced a greater later but not early ERP amplitude than neutral faces. This is consistent with previous studies in which individuals with social anxiety exhibited an enhanced early attention component in response to faces in general (Kolassa et al., 2009), but they showed a late overreaction to a specific negative expression (Hagemann et al., 2016). However, we did not observe an interaction between face and time or group. Our emotional Stroop task consisted of three types of expressions, and the effects of ABM training were found not only on trained disgust or neutral expressions but also on untrained fear. This may further suggest that training produces a kind of cross-expression transfer rather than a passive ease of reaction caused by long-term exposure to similar materials.
Perhaps more interestingly, we found a significant positive correlation between reduced social anxiety and LPP reductions in response to neutral faces. According to cognitive theories of social anxiety, those with the disorder do not only have attention bias but also show a tendency to misinterpret neutral social signals as threatening (Yoon et al., 2007). Some studies have reported that individuals with social anxiety have greater LPPs for threatening and also neutral face stimuli (Kujawa et al., 2015). The observed reduced LPP in response to neutral faces may indicate the elimination of interpretation bias. Given that negative interpretation of ambiguous information is a critical factor in deepening social distress and anxiety symptom maintenance (Alden & Taylor, 2004); the result can be highly instructive. This suggests that correcting a bias in interpretation may offer potential clinical benefits for social anxiety.
Limitations
Firstly, our participants were selected from a large sample pool of college students. Although their self-reported scores reached the specific level of social anxiety and they declared no history of psychiatric visits. These participates were not clinically diagnosed by psychiatrist. In addition, in our research, there is a time interval (12 months) between the recruitment of participants in the waiting group and most of participants in the AMP and ACC groups. Although three groups were completely matched in terms of demography and recruitment standards, timing of observations may affect the results; thus, more rigorous clinical trials are expected.
Secondly, the avoidance subscale of LSAS was excluded from the training evaluation for its inferior suitability for short-term tracking; however, the lack of data regarding behavior avoidance across training hindered us from assessing the objective effects of attention training. Future studies may add more evaluation indicators to the training process to track the dynamic changes of training benefits.
Finally, due to the lack of collection of subjective evaluation of faces in the emotional Stroop task, we cannot provide a direct understanding but some speculation to the corresponding ERPs changes. Future research needs to add subjective assessment to the emotional stimulus to make the neurological changes more clearly explained.
Conclusion
We found a dissociable mechanism underlying ABM in social anxiety. While the unique manipulation that is characteristic of ABM mainly modulated the early stage of attentional processing, the actual clinical benefits of attention training may be derived from later cognitive-affective processes.
Funding
This research was supported by National Nature Science Foundation of China [NSFC 31671136, 31530031].
Conflict of interest
None declared.
Supplementary Material
References
- Alden L.E., Taylor C.T. (2004). Interpersonal processes in social phobia. Clinical Psychology Review, 24, 857–82. [DOI] [PubMed] [Google Scholar]
- Amir N., McNally R.J., Riemann B.C., Burns J., Lorenz M., Mullen J.T. (1996). Suppression of the emotional Stroop effect by increased anxiety in patients with social phobia. Behaviour Research and Therapy, 34, 945–8. [DOI] [PubMed] [Google Scholar]
- Amir N., Elias J., Klumpp H., Przeworski A. (2003). Attentional bias to threat in social phobia: facilitated processing of threat or difficulty disengaging attention from threat? Behaviour Research and Therapy, 41, 1325–35. [DOI] [PubMed] [Google Scholar]
- Amir N., Weber G., Beard C., Bomyea J., Taylor C.T. (2008). The effect of a single-session attention modification program on response to a public-speaking challenge in socially anxious individuals. Journal of Abnormal Psychology, 117, 860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N., Beard C., Taylor C.T., Klumpp H., Elias J., Bums M., Chen X. (2009). Attention training in individuals with generalized social phobia: a randomized controlled trial. Journal of Consulting and Clinical Psychology, 77, 961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N., Bomyea J., Beard C. (2010). The effect of single-session interpretation modification on attention bias in socially anxious individuals. Journal of Anxiety Disorders, 24, 178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrington C.M., Carr T.H., Mayer A.R., Rao S.M. (2000). Neural mechanisms of visual attention: object-based selection of a region in space. Journal of Cognitive Neuroscience, 12, 106–17. [DOI] [PubMed] [Google Scholar]
- Askew C., Hagel A., Morgan J. (2015). Vicarious learning of children’s social-anxiety-related fear beliefs and emotional Stroop bias. Emotion, 15, 501–10. [DOI] [PubMed] [Google Scholar]
- APA (2013). Diagnostic and statistical manual of mental disorders (DSM-5) (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Baker S.L., Heinrichs N., Kim H.J., Hofmann S.G. (2002). The Liebowitz social anxiety scale as a self-report instrument: a preliminary psychometric analysis. Behaviour Research and Therapy, 40, 701–15. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y. (2010). Research review: attention bias modification (ABM): a novel treatment for anxiety disorders. Journal of Child Psychology and Psychiatry, 51, 859–70. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M. J. IJzendoorn M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin 133, 1–24. [DOI] [PubMed] [Google Scholar]
- Batty M., Taylor M.J. (2003). Early processing of the six basic facial emotional expressions. Brain Research. Cognitive Brain Research, 17, 613–20. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Ball R., Ranieri W. (1996). Comparison of beck depression inventories -ia and -ii in psychiatric outpatients. Journal of Personality Assessment, 67, 588. [DOI] [PubMed] [Google Scholar]
- Biehl M., Matsumoto D., Ekman P., Hearn V., Heider K., Kudoh T., Ton V. (1997). Matsumoto and Ekman’s Japanese and Caucasian Facial Expressions of Emotion (JACFEE): reliability data and cross-national differences. Journal of Nonverbal Behavior, 21, 3–21. [Google Scholar]
- Britton J.C., Suway J.G., Clementi M.A., Fox N.A., Pine D.S., Bar-Haim Y. (2015). Neural changes with attention bias modification (abm) for anxiety: a randomized trial. Social Cognitive and Affective Neuroscience, 10, 913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot J.F., De Ruiter C., Kindt M. (1999). Processing bias in anxious subjects and repressors, measured by emotional Stroop interference and attentional allocation. Personality & Individual Differences, 26(5), 777–93. [Google Scholar]
- Browning M., Holmes E.A., Murphy S.E., Goodwin G.M., Harmer C.J. (2010). Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biological Psychiatry, 67(10), 919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner J.D., Maner J.K., Schmidt N.B. (2010). Difficulty disengaging attention from social threat in social anxiety. Cognitive Therapy and Research, 34, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Koster E.H.W. (2010). Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clinical Psychology Review, 30, 203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Voogd E.L., Wiers R.W., Prins P.J.M., Salemink E. (2014). Visual search attentional bias modification reduced social phobia in adolescents. Journal of Behavior Therapy and Experimental Psychiatry, 45, 252–9. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Dennis T.A., O'Toole L. (2014). Mental health on the go: effects of a gamified attention bias modification mobile application in trait anxious adults. Clinical Psychological Science: A Journal of the Association for Psychological Science, 2, 576–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis-Tiwary T.A., Egan L.J., Babkirk S., Denefrio S. (2016). For whom the bell tolls: neurocognitive individual differences in the acute stress-reduction effects of an attention bias modification game for anxiety. Behaviour Research and Therapy, 77, 105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D., Reed M.A. (2002). Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology, 111, 225–36. [DOI] [PubMed] [Google Scholar]
- Eldar S., Bar-Haim Y. (2010). Neural plasticity in response to attention training in anxiety. Psychological Medicine, 40, 667–77. [DOI] [PubMed] [Google Scholar]
- Eysenck M.W., Derakshan N. (2011). New perspectives in attentional control theory. Personality and Individual Differences, 50, 955–60. [Google Scholar]
- Fields E.C., Kuperberg G.R. (2012). It’s all about you: an ERP study of emotion and self-relevance in discourse. NeuroImage, 62, 562–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganis G., Smith D., Schendan H.E. (2012). The N170, not the P1, indexes the earliest time for categorical perception of faces, regardless of interstimulus variance. NeuroImage, 62, 1563. [DOI] [PubMed] [Google Scholar]
- Gilboa-Schechtman E., Foa E.B., Amir N. (1999). Attentional biases for facial expression in social phobia: the face-in-the-crowd paradigm. Cognition and Emotion, 13, 305–18. [Google Scholar]
- Gong X., Huang Y.X., Wang Y., Luo Y.J. (2011). Revision of the Chinese facial affective picture system. Chinese Mental Health Journal. [Google Scholar]
- Gorlin E.I., Teachman B.A. (2015). Threat interference biases predict socially anxious behavior: the role of inhibitory control and minute of stressor. Behavior Therapy, 46, 493–509. [DOI] [PubMed] [Google Scholar]
- Hagemann J., Straube T., Schulz C. (2016). Too bad: bias for angry faces in social anxiety interferes with identity processing. Neuropsychologia, 84, 136–49. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Moser J.S., Simons R.F. (2006). Attending to affect: appraisal strategies modulate the electrocortical response to arousing pictures. Emotion, 6, 517–22. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Dunning J.P., Foti D. (2009). Motivated and controlled attention to emotion: time-course of the late positive potential. Clinical Neurophysiology, 120, 505–10. [DOI] [PubMed] [Google Scholar]
- Hajcak G., MacNamara A., Olvet D.M. (2010). Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology, 35, 129–55. [DOI] [PubMed] [Google Scholar]
- Hakamata Y., Lissek S., Bar-Haim Y., et al. (2010). Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry, 68, 982–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion L.S., Ruscio A.M. (2011). A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin, 137, 940–58. [DOI] [PubMed] [Google Scholar]
- Heeren A., Lievens L., Philippot P. (2011). How does attention training work in social phobia: disengagement from threat or re-engagement to non-threat? Journal of Anxiety Disorders, 25, 1108–15. [DOI] [PubMed] [Google Scholar]
- Hunkin L.M. (2014). Engagement with angry faces during attentional bias modification: Insights from the N2pc In: Cognitive and Behavioural Neuroscience, Victoria University of Wellington. [Google Scholar]
- Imbir K.K., Spustek T., Duda J., Bernatowicz G., Zygierewicz J. (2017). N450 and LPC event-related potential correlates of an emotional Stroop task with words differing in valence and emotional origin. Frontiers in Psychology, 8, 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier R.J., Taylor M.J. (2004). N170 or N1? Spatiotemporal differences between object and face processing using ERPs. Cerebral Cortex, 14, 132–42. [DOI] [PubMed] [Google Scholar]
- Jeffreys D.A. (1989). A face-responsive potential recorded from the human scalp. Experimental Brain Research, 78, 193–202. [DOI] [PubMed] [Google Scholar]
- Jeffreys D.A. (1996). Evoked potential studies of face and object processing. Visual Cognition, 3, 1–38. [Google Scholar]
- Joyce C., Rossion B. (2005). The face-sensitive N170 and VPP components manifest the same brain processes: the effect of reference electrode site. Clinical Neurophysiology, 116, 2613–31. [DOI] [PubMed] [Google Scholar]
- Khanna M.M., Badurabrack A.S., Mcdermott T.J., Shepherd A., Heinrichsgraham E., Pine D.S., et al. (2015). Attention training normalises combat-related post-traumatic stress disorder effects on emotional stroop performance using lexically matched word lists In: Cognition & Emotion. [DOI] [PMC free article] [PubMed]
- Klumpp H., Amir N. (2010). Preliminary study of attention training to threat and neutral faces on anxious reactivity to a social stressor in social anxiety. Cognitive Therapy and Research, 34, 263–71. [Google Scholar]
- Kolassa I.T., Kolassa S., Bergmann S., Lauche R., Dilger S., Miltner W.H.R., Musial F. (2009). Interpretive bias in social phobia: an ERP study with morphed emotional schematic faces. Cognition & Emotion, 23(1), 69–95. [Google Scholar]
- Kujawa A., MacNamara A., Fitzgerald K.D., Monk C.S., Phan K.L. (2015). Enhanced neural reactivity to threatening faces in anxious youth: evidence from event-related potentials. Journal of Abnormal Child Psychology, 43, 1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary M.R. (1983). A brief version of the fear of negative evaluation scale. Personality & Social Psychology Bulletin, 9, 371–5. [Google Scholar]
- Lee T.H., Lim S.L., Lee K., Kim H.T., Choi J.S. (2009). Conditioning-induced attentional bias for face stimuli measured with the emotional Stroop task. Emotion, 9, 134–9. [DOI] [PubMed] [Google Scholar]
- Li S.W., Tan J.Q., Qian M.Y., Liu X.H. (2008). Continual training of attentional bias in social anxiety. Behaviour Research and Therapy, 46, 905–12. [DOI] [PubMed] [Google Scholar]
- Li H., Wei D., Browning M., Du X., Zhang Q., Qiu J. (2016). Attentional bias modification (abm) training induces spontaneous brain activity changes in young women with subthreshold depression: a randomized controlled trial. Psychological Medicine, 46(05), 909–20. [DOI] [PubMed] [Google Scholar]
- Ma J., Liu C., Chen X. (2016). Emotional modulation of conflict processing in the affective domain: evidence from event-related potentials and event-related spectral perturbation analysis. Scientific Reports, 6, 31278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod C., Mathews A., Tata P. (1986). Attentional bias in emotional disorders. Journal of Abnormal Psychology, 95, 15–20. [DOI] [PubMed] [Google Scholar]
- Mattick R.P., Clarke J.C. (1998). Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behaviour Research and Therapy, 36, 455–70. [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P., Dixon C., Fisher S., Twelftree H., Mcwilliams A. (2000). Trait anxiety, defensiveness and selective processing of threat: an investigation using two measures of attentional bias. Personality & Individual Differences, 28(6), 1063–77. [Google Scholar]
- Mogoase C., David D., Koster E.H. (2014). Clinical efficacy of attentional bias modification procedures: an updated meta-analysis. Journal of Clinical Psychology, 70, 1133–57. [DOI] [PubMed] [Google Scholar]
- Nortje C., Posthumus T. (2012). Scores on an emotional Stroop task after treatment of social anxiety disorder. Psychological Reports, 111, 461–71. [DOI] [PubMed] [Google Scholar]
- Ohman A., Mineka S. (2001). Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review, 108, 483–522. [DOI] [PubMed] [Google Scholar]
- Osinsky R., Wilisz D., Kim Y., Karl C., Hewig J. (2014). Does a single session of Attentional Bias Modification influence early neural mechanisms of spatial attention? An ERP study. Psychophysiology, 51, 982–9. [DOI] [PubMed] [Google Scholar]
- O'Toole L., Dennis T.A. (2012). Attention training and the threat bias: an ERP study. Brain and Cognition, 78, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pishyar R., Harris L.M., Menzies R.G. (2004). Attentional bias for words and faces in social anxiety. Anxiety Stress and Coping, 17, 23–36. [Google Scholar]
- Schmack K., Burk J., Haynes J.D., Sterzer P. (2016). Predicting subjective affective salience from cortical responses to invisible object stimuli. Cerebral Cortex, 26, 3453–60. [DOI] [PubMed] [Google Scholar]
- Schmidt N.B., Richey J.A., Buckner J.D., Timpano K.R. (2009). Attention training for generalized social anxiety disorder. Journal of Abnormal Psychology, 118, 5–14. [DOI] [PubMed] [Google Scholar]
- Schweizer S., Grahn J., Hampshire A., Mobbs D., Dalgleish T. (2013). Training the emotional brain: improving affective control through emotional working memory training. Journal of Neuroscience, 33, 5301–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens E.S., Weinberg A., Nelson B.D., Meissel E.E.E., Shankman S.A. (2018). The effect of panic disorder versus anxiety sensitivity on event-related potentials during anticipation of threat. Journal of Anxiety Disorders, 54, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchselvam R., Blechert J., Sheppes G., Rydstrom A., Gross J.J. (2011). The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biological Psychology, 87, 84–92. [DOI] [PubMed] [Google Scholar]
- Thomas S.J., Johnstone S.J., Gonsalvez C.J. (2007). Event-related potentials during an emotional Stroop task. International Journal of Psychophysiology, 63, 221–31. [DOI] [PubMed] [Google Scholar]
- Tokudome W., Wang G. (2012). Similarity dependency of the change in ERP component N1 accompanying with the object recognition learning. International Journal of Psychophysiology, 83, 102–9. [DOI] [PubMed] [Google Scholar]
- Wascher E., Hoffmann S., Sanger J., Grosjean M. (2009). Visuo-spatial processing and the N1 component of the ERP. Psychophysiology, 46, 1270–7. [DOI] [PubMed] [Google Scholar]
- Wheatley T., Weinberg A., Looser C., Moran T., Hajcak G. (2011). Mind perception: real but not artificial faces sustain neural activity beyond the N170/VPP. PLoS One, 6, e17960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.M.G., Mathews A., MacLeod C. (1996). The emotional stroop task and psychopathology. Psychological Bulletin, 120, 3–24. [DOI] [PubMed] [Google Scholar]
- Yoon K.L., Fitzgerald D.A., Angstadt M., McCarron R.A., Phan K.L. (2007). Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-tesla functional MRI study. Psychiatry Research-Neuroimaging, 154, 93–8. [DOI] [PubMed] [Google Scholar]
- Zhu X.R., Zhang H.J., Wu T.T., Luo W.B., Luo Y.J. (2010). Emotional conflict occurs at an early stage: evidence from the emotional face-word Stroop task. Neuroscience Letters, 478, 1–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


