Fig. 2: The function of Ltn1/Listerin in protein surveillance.
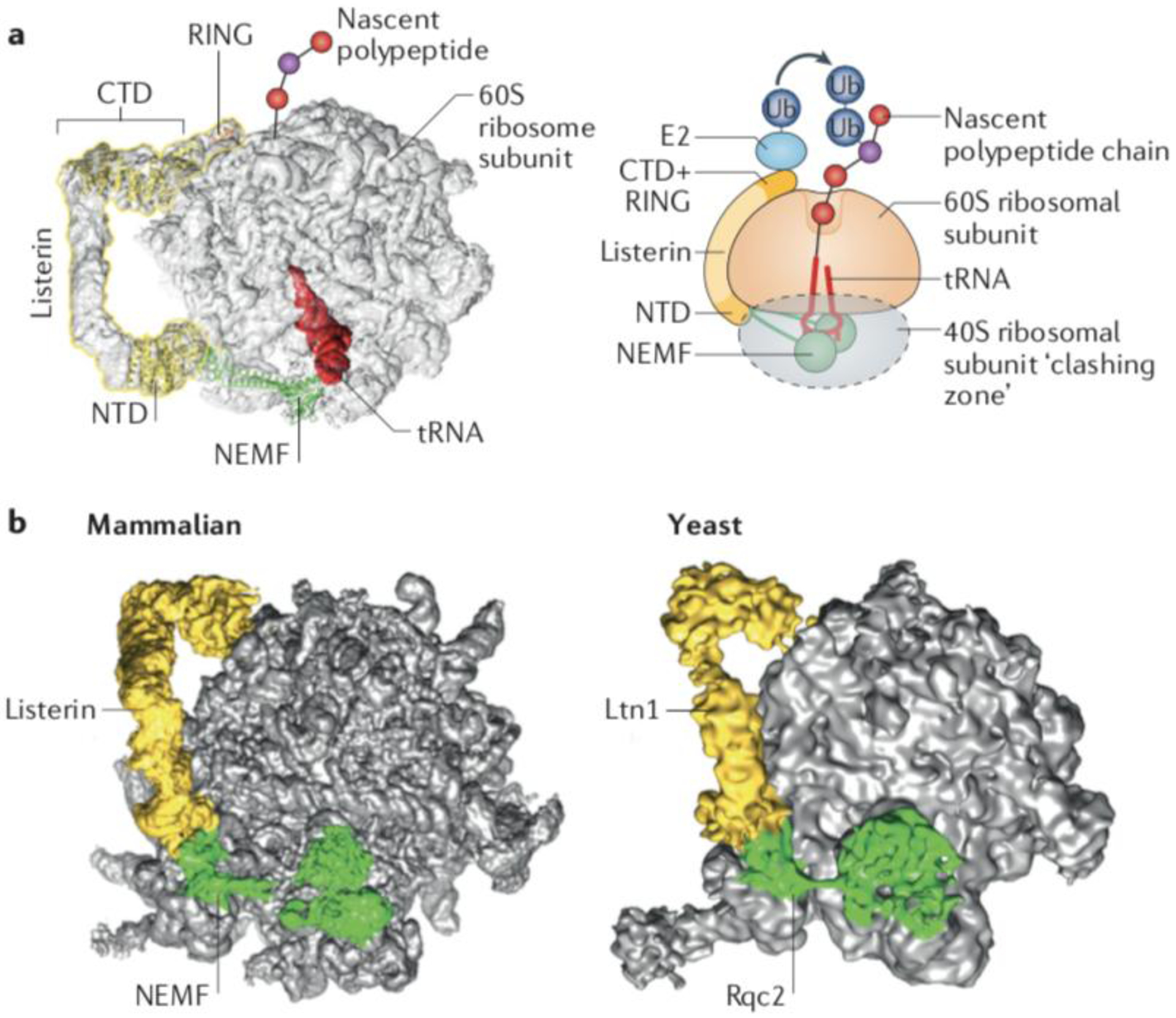
(a) Structural model of the ribosomal 60S subunit obstructed with peptidyl-tRNA and bound to the ribosomal quality control (RQC) complex subunits Listerin and NEMF. Superposition of cryo-EM volume of the RQC (EMDB 2832), molecular models of NEMF and the Listerin C-terminal Domain (CTD)22 (PDB 3J92), and the crystal structure of the Ltn1 N-terminal Domain (NTD)48 (PDB 5FG1). The cartoon on the right is a representation of the 60S subunit bond to the peptidyl-tRNA, Listerin/Ltn1 and NEMF/Rqc2. The conserved N-terminal and C-terminal domains of Ltn1, separated by a variable middle linker containing HEAT/ARM repeats are indicated. The C-terminal Ltn1 RING domain recruits E2~Ub to ubiquitylate nascent chains. The dotted grey oval indicates the space that would be occupied by the 40S subunit if present, to indicate steric clashes that would occur with Rqc2/NEMF and the Ltn1/Listerin NTD. (b) Comparison of the RQC structures from yeast (EMDB 2797)20 and mammals (EMDB 2832)22.
(Figures courtesy of H. Paternoga)
