Fig. 3: CAT tail synthesis and functions.
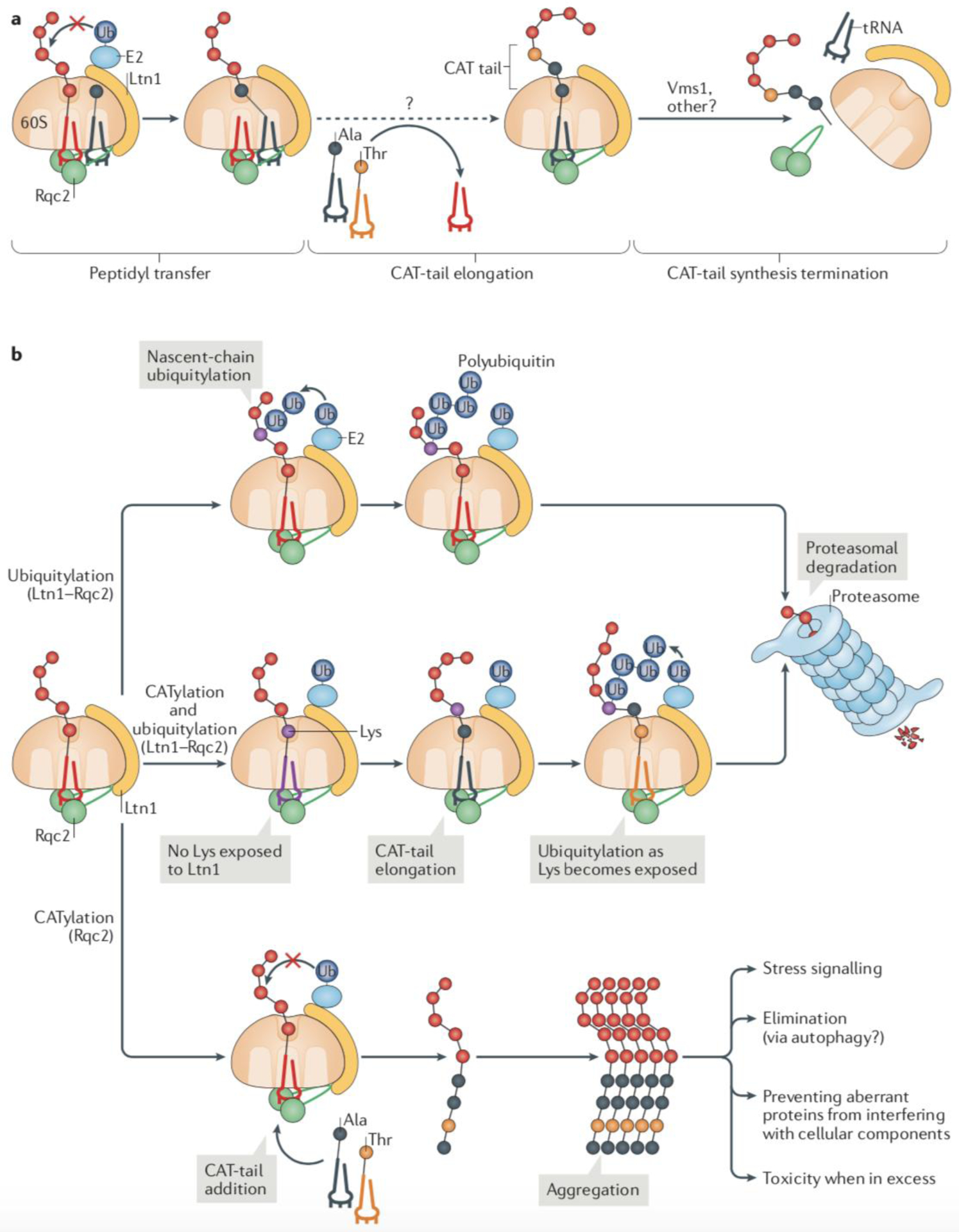
(a) The ribosome-associated protein quality control (RQC) complex subunit Rqc2 recognizes ribosomal 60S subunits that are obstructed by a peptidyl-tRNA by simultaneously binding to components of the 60S subunit and the tRNA. When ubiquitylation of the nascent polypeptide by Ltn1 fails, Rqc2 can extend the trapped nascent polypeptide with a C-terminal tail composed of Ala and Thr residues (CAT tail). The reaction is mediated by the direct recruitment of charged tRNA-Ala and tRNA-Thr by Rqc2 and occurs without an mRNA template. The Vms1 protein can terminate CAT tail synthesis by releasing the P-site tRNA and presumably promoting Rqc2 dissociation from the complex. (b) Alternative fates of RQC substrates. The canonical RQC pathway of Ltn1-mediated ubiquitylation of nascent polypeptides is kinetically preferred, provided that Lys ubiquitylation sites on nascent chains are readily accessible (top). In this pathway, Rqc2 functions in recruiting and stabilizing Ltn1 in the complex. An alternative pathway takes place when ubiquitylation is compromised (middle). In this pathway, Rqc2 catalyses the elongation of a CAT tail, which can result in the exposure of Lys residues that would otherwise be hidden in the ribosomal exit tunnel. The increased accessibility to Lys residues enables ubiquitylation by Ltn1. However, when ubiquitylation fails altogether (bottom), CAT tail-modified nascent chains form aggregates, which can have different fates and effects in cellular function, including stress signalling.
