Figure 4: RQC on the endoplasmic reticulum and mitochondrial membranes.
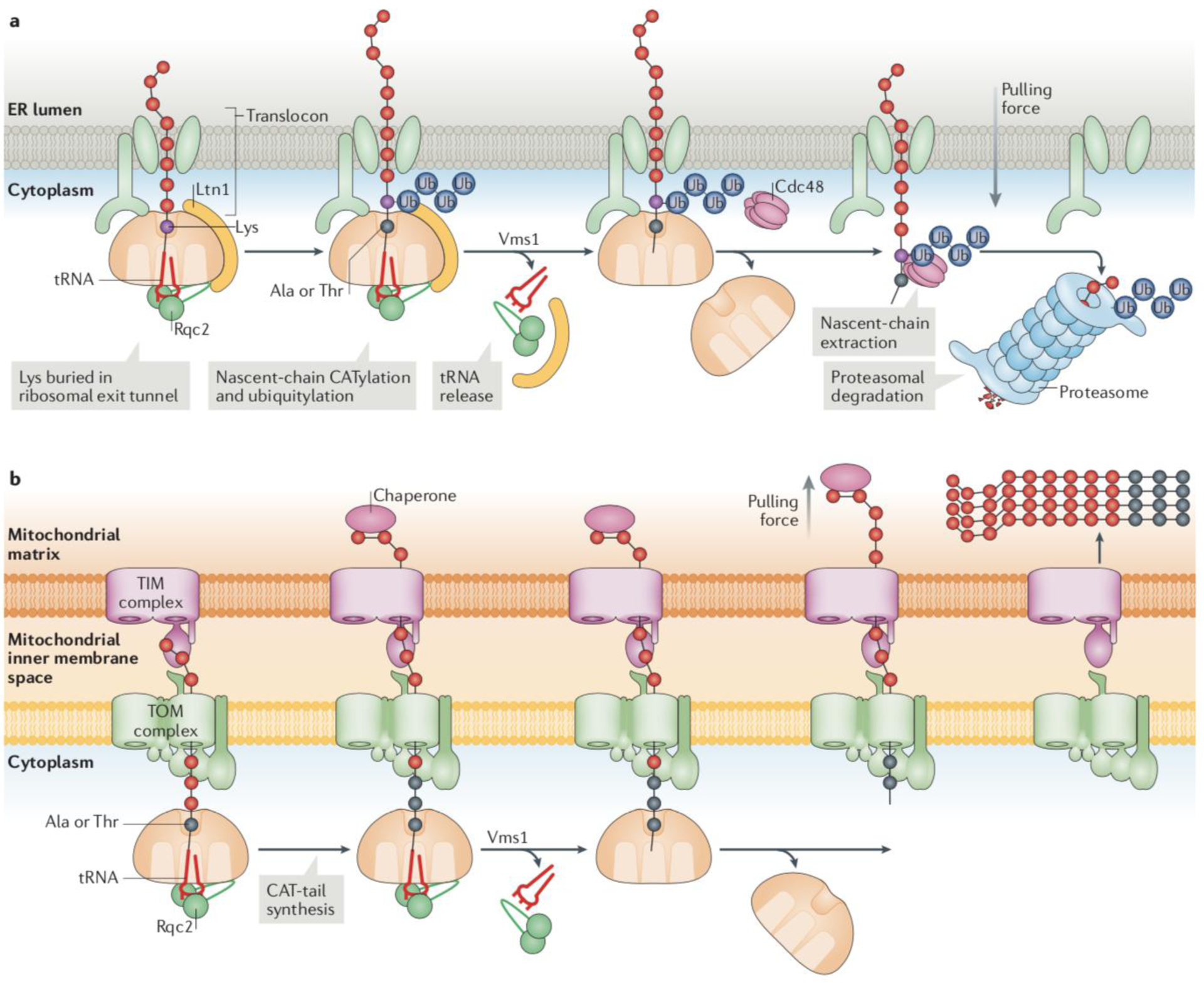
Ribosomes can stall while translating proteins destined to different subcellular compartments, such as the cytosol, mitochondria and the endoplasmic reticulum (ER). (a) ER-RQC: hypothetical model for RQC at ER-associated ribosomes. On nascent chains obstructing the 60S ribosomal subunit on organellar surfaces, Ltn1 only has limited access to Lys residues, within a short segment of cytosolic-exposed polypeptide sequence immediately outside the exit tunnel. The nascent chain segments buried in the ribosomal exit tunnel or the translocon, or inside the organelle, are not accessible to ubiquitylation. However, Lys residues hidden in the ribosomal exit tunnel can be exposed to Ltn1 through Rqc2-mediated nascent chain CATylation. Cdc48 is recruited to extract the ubiquitylated nascent chain and deliver it to the proteasome for degradation in the cytosol, after Vms1 catalyses nascent chain release from the tRNA. (b) Mito-RQC: Example of Ltn1 dysfunction on the mitochondrial surface. In the absence of nascent chain ubiquitylation, nascent chains are CATylated by Rqc2 and released into the mitochondrial matrix by Vms1. The directional pulling force by a mitochondrial chaperone (in pink) is indicated. In the matrix, moderate levels of CAT tail-dependent aggregates can be handled by the protein homeostasis machinery. However, if both Ltn1- and Vms1-mediated processes fail, CATylation proceeds for an extended period, resulting in the accumulation of larger aggregates which mitochondria may have more difficulty in eliminating, and which can interfere with the function of the organelle66.
