Figure 1.
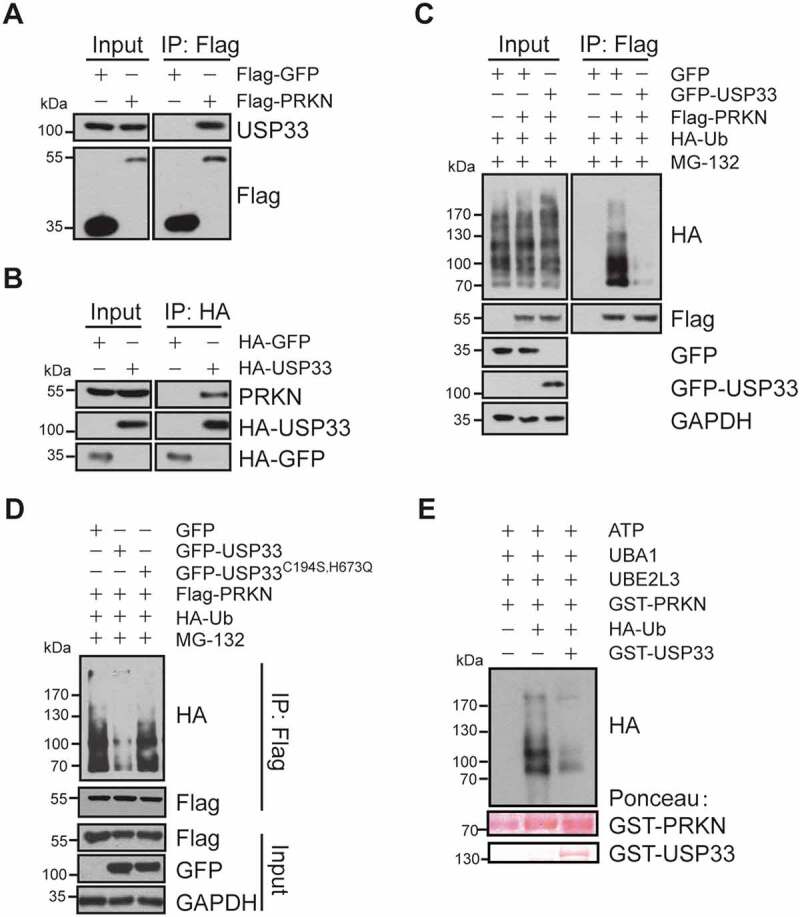
USP33 interacts with PRKN. (A) Endogenous USP33 protein was detected in anti-Flag immunoprecipitate from cell lysate of HEK293 cells overexpressing Flag-PRKN by western blotting. (B) Endogenous PRKN protein was verified in anti-HA immunoprecipitate from the lysate of HA-USP33 transfected HEK293 cells. HA-GFP was used for negative control. (C) Decreased ubiquitination level of PRKN in HEK293 cells overexpressing GFP-USP33. HEK293 cells were co-transfected with Flag-PRKN, HA-Ub and GFP-USP33, then treated with MG-132 for 3 h. Flag-PRKN was pulled-down with anti-Flag antibody and the PRKN ubiquitination level was detected with anti-HA antibody by western blotting. (D) Defective deubiquitination activity of USP33 mutant (USP33C194S,H673Q mutant) on PRKN. HEK293 cells were co-transfected with Flag-PRKN, HA-Ub and GFP-USP33 or GFP-USP33C194S,H673Q mutant, then treated with 10 μM MG-132 for 3 h. PRKN ubiquitination level was examined in Flag immunoprecipitates with anti-HA by western blotting. (E) USP33 removes PRKN Ub conjugates by in vitro ubiquitination assay. GST-PRKN and GST-USP33 levels stained with Ponceau were included.
