Figure 6.
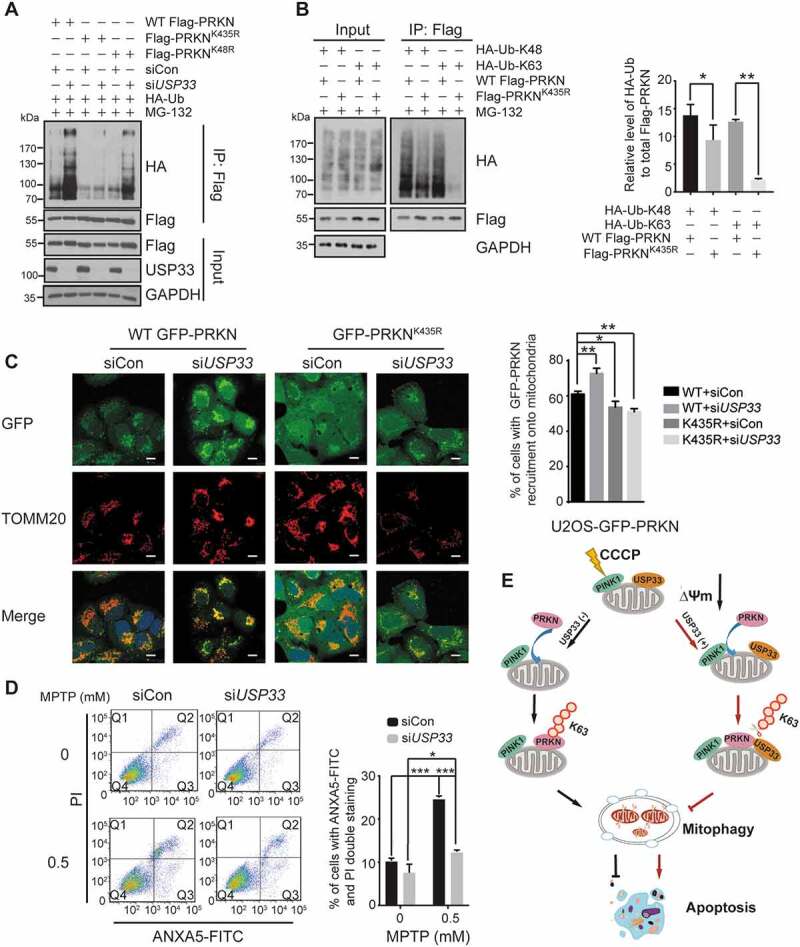
USP33 regulates PRKN ubiquitination at Lys435 and its silencing protects SH-SY5Y cells from the neurotoxin MPTP. (A) Lysine sites on PRKN deubiquitinated by USP33. U2OS cells were transfected with the indicated constructs for 24 h, then with siCon or USP33 siRNA1 + 2 (siUSP33) for 48 h. PRKN ubiquitination level in Flag immunoprecipitates was examined with anti-HA antibody by western blotting. (B) Levels of K48- and K63-linked Ub at Lys435. HEK293 cells were transfected with Flag-tagged WT PRKN or K435R mutant with HA-Ub-K48 or HA-Ub-K63 for 36 h, and harvested after 10 μM MG-132 treatment. The densities of PRKN ubiquitination were quantified using Image J software. The data represent the mean ± SD from three independent experiments. A one-way ANOVA and Student’s t test were performed for statistical analysis. *P < 0.05, **P < 0.01. (C) An enhanced recruitment of WT PRKN, but not K435R mutant, to depolarized mitochondria in USP33-depleted U2OS cells. U2OS cells stably expressing WT or K435-mutated PRKN were used. The images were captured using Leica TCS SP8 Confocal microscope. Scale bar: 10 μm. Percentage of cells with WT GFP-PRKN or GFP-PRKNK435R puncta colocalized with TOMM20 was quantified in siControl and USP33-depleted cells after 60 min of CCCP treatment. Over 100 cells were examined for each time-point, and data represents mean ± SD from three independent experiments. *P < 0.05, **P < 0.01, Student’s t test. (D) SH-SY5Y cells were transfected with siCon or USP33 siRNA1 + 2 (siUSP33) for 48 h, and then treated with 0.5 mM MPTP for 24 h. The cells were collected and stained with ANXA5-FITC and propidium iodide (PI) using the ANXA5/annexin V-FITC/PI Detection Kit (Beyotime, C1062M). The percentage of apoptotic cells were quantified by flow cytometry (BD FACSAria II). The data represents mean ± SD from three independent experiments. *P < 0.05 and ***P < 0.001, Student’s t test. (D) Model depicting the antagonizing effect of USP33 on PRKN-mediated mitophagy. USP33 as an OMM protein interacts with PRKN under mitochondrial depolarization and regulates the magnitude of PRKN-induced mitophagy mainly through removing K63-linked Ub chain from PRKN. USP33 deficiency leads a persistent elevation of K63-linked Ub conjugates on PRKN consequently stabilizing PRKN protein and augmenting mitophagy induction. Overall, USP33 depletion promotes an efficient clearance of damaged mitochondria and potently protects neuron-like cells from neurotoxin-induced apoptotic cell death.
