Figure 2. Characterization and validation of the electrochemical sensor for non-invasive cortisol analyses.
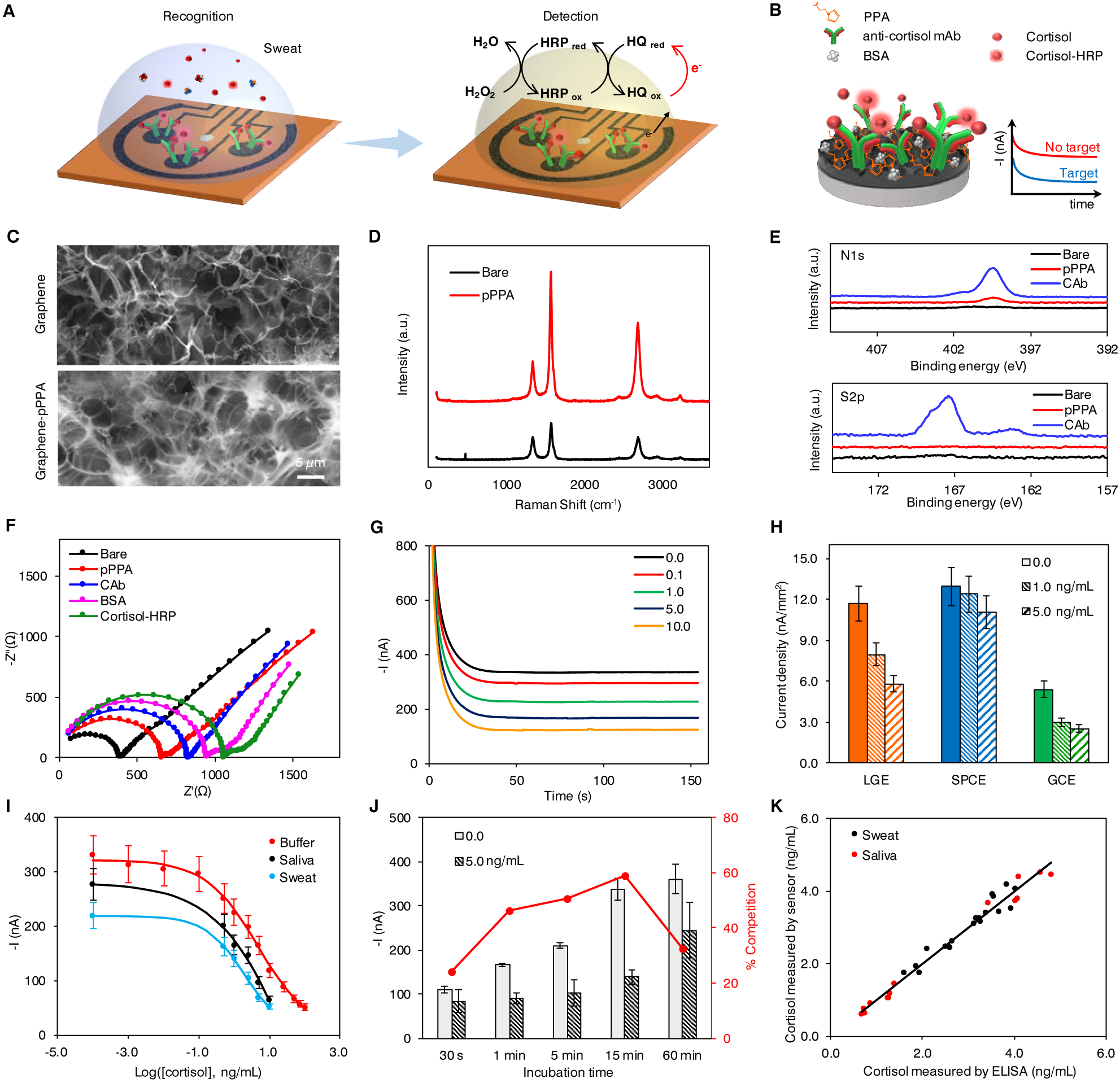
(A and B) Schematic of the electrochemical detection of cortisol in human sweat (A) and representation of the affinity-based electrochemical cortisol sensor construction and sensing strategy (B). HRP, horseradish peroxidase; HQ, hydroquinone; PPA, pyrrole propionic acid; BSA, bovine serum albumin; mAb, monoclonal antibody.
(C) Scanning electron microscopy (SEM) images of the graphene electrode surface before and after PPA polymerization.
(D and E) Raman spectra (D) and X-ray photoelectron spectra (E) of bare graphene electrode, and graphene electrodes modified with PPA (pPPA) and capture antibody (CAb).
(F) Nyquist plots of a graphene electrode in a 0.01 M PBS solution containing 2.0 mM of K4Fe(CN)6/K3Fe(CN)6 (1:1) after each surface modification step: bare graphene, electropolymerization of PPA (pPPA), capture antibody immobilization (CAb), blocking with BSA and incubation with enzyme-tagged cortisol (cortisol-HRP).
(G) Amperometric signals of the flexible graphene-based biosensors for 0.0–10.0 ng/mL cortisol in 0.01 M PBST, pH 7.4.
(H) Sensor performance of laser-induced graphene electrode (LGE) vs. screen printed carbon electrode (SPCEs) and glassy carbon electrodes (GCEs). Current densities were obtained from 0.0, 1.0, and 5.0 ng/mL cortisol solutions. Data are presented as mean ± standard deviation (SD) (n = 3).
(I) Full sigmoidal calibration curves constructed for cortisol in buffer, sweat and saliva. The sweat and saliva samples were collected from a healthy subject. Data are presented as mean ± SD (n = 3).
(J) Amperometric responses and percentage competition observed for 0.0 and 5.0 ng/mL cortisol with 30-second, 1-, 5-, 15-, and 60-minute incubation. Data are presented as mean ± SD (n = 3). (K) Validation of the flexible graphene-based biosensors toward cortisol monitoring in real samples with enzyme-linked immunosorbent assay (ELISA).
