Figure 3. Frontier molecular orbital energy diagram describing the photophysical processes affording NO2-Rosol an OFF-ON NIR fluorescence response upon its NTR activity-facilitated bioreductive activation.
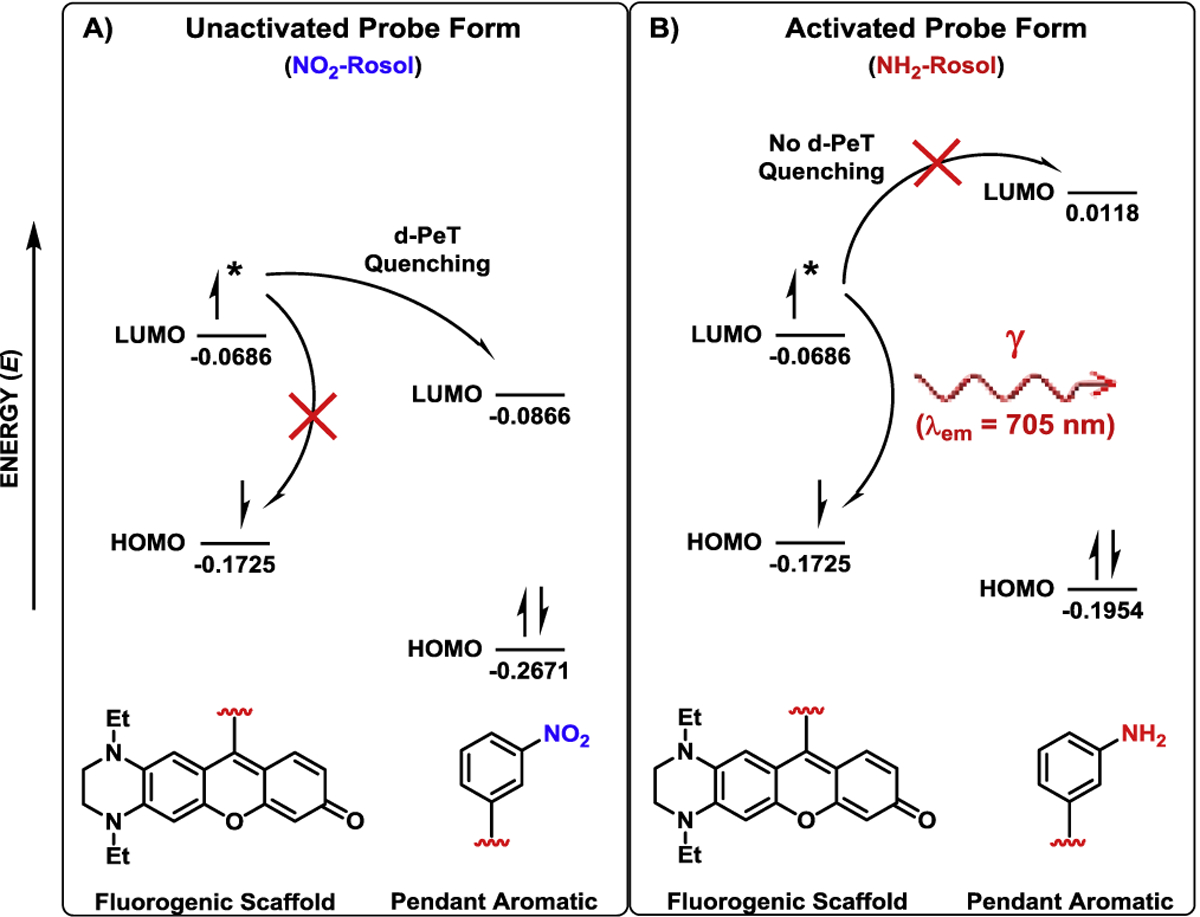
A) Favorable energetics between the LUMO of the fluorogenic scaffold and the LUMO of the pendant m-nitrobenzene permit the excited electron that momentarily occupies the LUMO of the former to undergo an intramolecular d-PeT to the LUMO of the latter, and thereby quenches the NIR fluorescence emission that the excited electron would otherwise afford via it subsequently relaxing from the LUMO of the pendant m-nitrobenzene in a non-radiative decay process. B) Unfavorable energetics between the LUMO of the fluorogenic scaffold and the LUMO of the pendant m-aminobenzene precludes a d-PeT fluorescence-quenching process from transpiring between the components, and thereby the excited electron momentarily occupying the LUMO of the fluorogenic scaffold can provide NIR fluorescence emission by relaxing back to the HOMO of the fluorogenic scaffold in a radiative decay process. LUMO = lowest unoccupied molecular orbital, HOMO = highest occupied molecular orbital. Asterisk (*) signifies that an electron from the HOMO of the fluorogenic scaffold exists in an excited electronic state in transitioning to the corresponding LUMO following upon photoexcitation. Molecular orbital energy values are provided in hartrees.
