Figure 1.
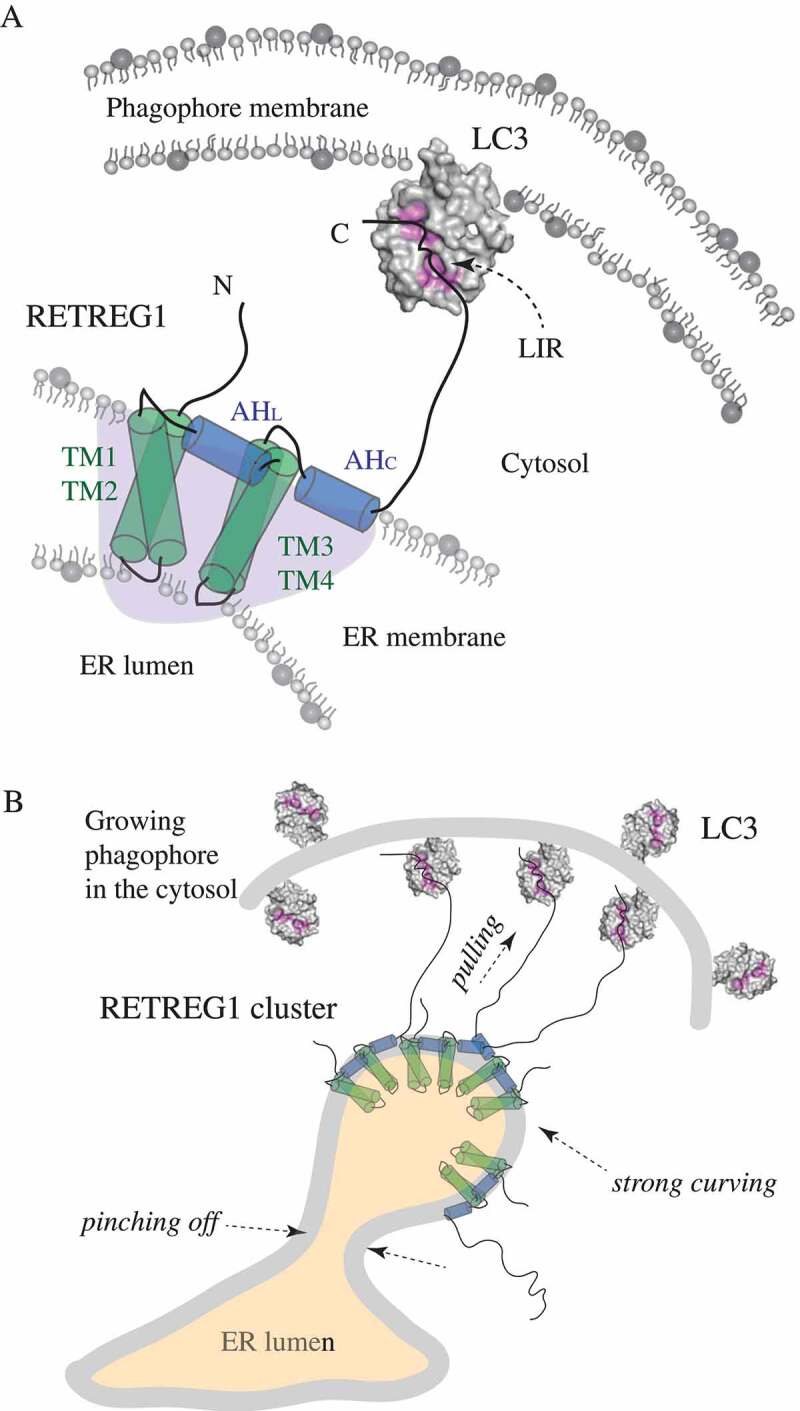
Schematic representation of the molecular mechanism of RETREG1 function. (A) A detailed zoom-in on a single molecule of the RETREG1 receptor embedded in the ER membrane via its RHD. The functional topology of the RETREG1 RHD, as revealed by MD simulations (redrawn here schematically based on ref [2].), is formed by several structural elements including the two transmembrane hairpins (TM1-TM2 and TM3-TM4) and two amphipathic helices (AHL and AHC). Together these elements adopt a wedge shape (light purple shading) of a single RHD molecule. The disordered N and C termini protrude into the cytosol. The C terminus of RETREG1 carries the LIR motif (FELL) that binds to the hydrophobic pockets (highlighted in pink) of LC3 (PDB ID: 4ZDV) that is conjugated to PE on the phagophore membrane. (B) The molecular mechanism of RETREG1 function in selective reticulophagy as proposed by Bhaskara et al. The wedge-shaped RHDs of RETREG1 cluster together and form an inverted-pyramid-like shape, which strongly curves the ER membrane. The C terminal LIR motif of each RETREG1 molecule binds to LC3 on the concave side of the phagophore. Pulling forces by the multiple long disordered C termini of RETREG1 molecules along with strong curving forces by the clustered RHDs facilitate ER vesicle budding and subsequent pinching off. This leads to fragmentation of the ER, which is necessary for engulfing by the phagophore.
