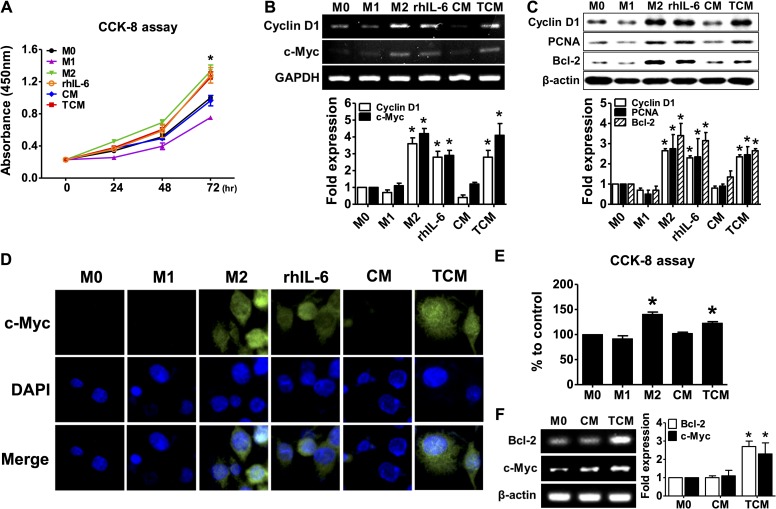
Fig 5. Proliferation of macrophages in response to conditioned medium of human prostate epithelial cells (RWPE-1) stimulated with T. vaginalis.
THP-1 monocytes were treated with 100 nM PMA for 24 hr to obtain M0 macrophages and then cultured in complete medium for 72 hr. The cells were then treated with 100 ng/ml LPS and 20 ng/ml IFN-γ for M1 macrophages, 20 ng/ml IL-4 and 20 ng/ml IL-13 for M2 macrophages, 5 ng/ml IL-6, CM, or TCM for 72 hr. Human peripheral blood mononuclear cells (PBMCs) incubated with 50 ng/ml M-CSF for 2 hr to allow monocyte adhesion. Non-adherent cells were removed and adherent monocytes were treated with 50 ng/ml M-CSF for 6 days for macrophage differentiation, and then cultured with CM, or TCM for 48 hr. (A) Proliferation of THP-1-derived macrophages was measured by CCK-8 assay. (B) mRNA expression of cyclin D1 and c-Myc was determined by RT-PCR. Densitometry of mRNA bands were quantified by three independent experiments. (C) Expression of PCNA, cyclin D1 and Bcl-2 was determined by western blot. Graph represent densitometric analysis (means of three independent western blot experiments). (D) Immunofluorescence assays were performed using an anti-c-Myc primary antibody and an anti-rabbit Alexa Fluor-594-labeled secondary antibody (DNA stained with DAPI/blue) and images were selected from five random fields. (E) Proliferation of human PBMCs-derived macrophages was measured by CCK-8 assay. (F) Expression of Bcl-2 and c-Myc was determined by RT-PCR. Densitometry of mRNA bands were quantified by three independent experiments. Data are means ± SD of three independent experiments. *p<0.05 versus THP-1-derived macrophages or HMDM (M0). CM: conditioned medium of RWPE-1 alone, TCM: conditioned medium of RWPE-1 stimulated with T. vaginalis.