Abstract
Purpose
Type 2 diabetes mellitus (T2DM) is a disease with a steadily increasing incidence throughout the world. Some molecules regulating the innate immune responses such as toll-like receptor 4 (TLR4) have shown to be involved in late diabetic complications. This study aimed to investigate the association of TLR4 gene polymorphisms with clinicopathological aspects of T2DM in the Iranian population.
Patients and Methods
Two TLR4 896A>G and 1196C>T polymorphisms were assessed in 100 T2DM patients and 100 healthy controls using sequence-specific primers PCR. Demographic, anthropometric, and biochemical parameters were obtained from the participants.
Results
After logistic regression, in 1196C>T, a significant association was shown between diabetic nephropathy (DN) and CT genotype (P= 0.04, OR= 4.35, CI= (1.04–18.1)). TG level has increased significantly in both T2DM and control subjects with CT genotype (P= 0.027, OR= 1.005, 95% CI= (1.001–1.01)). For 896A>G variant, a significant association was also detected between AG genotype and increased oral glucose tolerance test (OGTT) level (P= 0.048, OR= 1.003, 95% CI= (1.00–1.005)).
Conclusion
Although minor alleles of 1196C>T and 896A>G variants have not directly been associated with type 2 diabetes, by involving in the dysregulation of serum TG and blood sugar levels, they might increase the risk of DN.
Keywords: type 2 diabetes mellitus, T2DM, diabetic nephropathy, DN, triglyceride, TG, oral glucose tolerance test, OGTT, 896A>G, 1196C>T
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic and multifactorial metabolic disorder which is characterized by chronic hyperglycemia.1,2 World Health Organization-Diabetes country profiles stated 10.3% prevalence of diabetes in Iran in 2016.3 Haghdoost et al have been reported that the prevalence of type 2 diabetes appears higher in Iran than in other developing countries.4,5 T2DM and its accompanied serious complication including cardiovascular diseases, neuropathy and diabetic nephropathy are among the growing global health challenges.1 Several risk factors have been associated with the onset of T2DM, including family history, ethnicity, obesity, and abnormal serum lipid levels.6–8
Low-grade inflammation9,10 and innate immune response have been implicated as important pathogenic determinants of DM and late diabetic complications.11–13 Among innate immune receptors, Toll-like receptor 4 (TLR4) is one of the key receptors forming an initial line of defense.14,15 The TLR4 gene is Located on 9q33.1. TLR4 is distributed in many cells including macrophages, endothelial cells, brain, gut, liver, pancreas, muscle and adipose tissues.14 TLR4 is the receptor for structurally diverse molecules such as bacterial lipopolysaccharide (LPS),16 endogenous ligands; oxidized LDL, heat shock proteins (HSP) 60 and 70, fibrinogen, and fibronectin.17 Recent studies showed that TLR4 up-regulation18 and activation have been associated with the inflammatory response in obesity and insulin resistance diabetic patients.19–21 Moreover, there is a growing interest in the role of TLR4 polymorphisms, especially 896A>G and 1196C>T in T2DM progression and its complication including nephropathy which had inconsistent results.21–24 Due to the lack of information concerning the TLR4 polymorphisms in Iranian T2DM patients and the higher prevalence of T2DM in Iranian population compared with other developing countries,4,5 we opted to explore the potential association of two TLR4 896A>G and 1196C>T polymorphisms with predisposition to T2DM.
Materials and Methods
Subjects
A total of 100 T2DM patients (M: F = 27:73) (Mean age ± SD= 48.2 ± 7.14) and 100 age- and sex-matched healthy control subjects (M: F = 29:71) (Mean age ±SD = 47.3 ± 5.95) from Payambar Azam medical educational complex, Bandar Abbas, Hormozgan, Iran have been enrolled in this study. The written informed consent was obtained from each participant before enrollment in the study. Healthy subjects had been checked by the physician in the department of health center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran. After 12 hrs of fasting, 7 mL of venous blood was taken from each participant. A normal fasting blood sugar level less than 100 mg/dL was referred for control without diabetes. The exclusion criteria were type 1 diabetes, severe peripheral vascular disease, and pregnancy. Nephropathy inclusion criteria have been determined based on American Diabetes Association (ADA).25 The persistent presence of elevated urinary albumin excretion (albuminuria), low-estimated glomerular filtration rate (eGFR), or other manifestations of kidney damage in persons with T2DM, were eligible for inclusion. All patients’ clinical features were accredited by the specialist. The clinical characteristics of the samples are shown in Table 1. Additional information for diabetic patients with or without nephropathy are shown in Supplementary Table 1. All biochemical parameters (FBS, TG, Cholesterol, HDL and LDL) were measured using Pars Azmun kit (Pars Azmun, Tehran, Iran). The study protocol was approved by the ethics committee (HUMS.REC.1394.85) of Hormozgan University of Medical Sciences and the study was conducted in accordance with the Declaration of Helsinki. We calculated the sample size using the open-source epidemiologic statistics for public health software, version 3.01. The power of the study was estimated greater than 80% to detect significant effects with the odds ratio greater than 4 for the minor allele frequency (around 5–6%) of rs4986790.
Table 1.
Clinical Characteristics of the Study Samples
| Case (n=100) | Control (n=100) | P | |
|---|---|---|---|
| Age | 50 (42.5–53) | 46 (43–50) | 0.36 |
| Male/Female (n) | 29/71 | 30/70 | 0.75 |
| Hypertension (n) | 37 | – | – |
| BMI | 25 (23−29) | 24 (21–25) | <0.001 |
| Chol (mg/dL) | 166.8 ± 37.6 | 191.3 ± 33 | <0.001 |
| HDL (mg/dL) | 42 (36.5–46) | 35.5 (40–64) | <0.001 |
| LDL (mg/dL) | 88.5 (72–111.5) | 97 (81.5−105) | 0.54 |
| TG (mg/dL) | 149.5 (116.5–195.5) | 100 (71–133) | <0.001 |
| FBS (mg/dL) | 196.7 ± 74.1 | 97 ± 11.02 | <0.001 |
| HbA1C (%) | 8.8 ± 2 | Not evaluated | – |
| OGTT (mg/dL) | 236.2 ± 98.4 | Not evaluated | – |
| T2DM duration(y) | 9.5 ± 5.3 | – | – |
| Statin therapy (n) | 49 | – | – |
| Nephropathy (n) | 11 | – | – |
| Family history IHD (n) | 37 | – | – |
Notes: Parametric values are presented as mean ± standard deviation and non-parametric data are presented as median (Q1-Q3). Data were evaluated using χ2-test for sex; Mann–Whitney U-test for Age, BMI, HDL, LDL, and TG; t-test for the other variables. P < 0.05 was considered as statistically significant.
Abbreviations: OGTT, oral glucose tolerance test; IHD, ischemic heart disease.
DNA Extraction and PCR Methods
DNA was extracted from blood cells using a genomic DNA isolation kit (PrimePrep, Genetbio, Korea). The concentration of extracted DNA was determined by Nanodrop spectrophotometer (NanoDrop 2000c, Thermo Fisher Scientific, USA). TLR4 variants including: 896A>G (GENE ID: rs4986790, HGVS:NC_000009.12:g.117713024A>G) and 1196C>T (GENE ID: rs4986790, HGVS:NC_000009.12:g.117713024A>G) amplification was done by Single Specific Primer-Polymerase Chain Reaction (SSP-PCR) assay. Two independent reactions were performed for each sample. Specific primers26 are shown in Table 2. HLA-DRB1 primers were used as an internal positive control. SSP-PCR mixture contained: 12.5 μL Taq 2x Master Mix Red (Ampliqon, Denmark), 2 μL of each primer pair (stock concentration of 10 μM), 1 μL of each internal control primer pair, 3 μL of sample DNA: (10–500 ng), and sterile double-distilled water to a final volume of 25 μL. The following touchdown PCR amplification condition was used for both of TLR4 variants:
Table 2.
The Sequence of Primers Used for SSCP-PCR Analysis of TLR4 Gene Polymorphisms
| Primer Sequence | Product Size (bp) | |
|---|---|---|
| TLR4 896A>G. F | 5’-TTAGACTACTACCCCGATGA-3’ | 307 |
| TLR4 896A>G. F’ | 5’-TTAGACTACTACCCCGATGG-3’ | |
| TLR4 896A>G. R | 5’-CACTTTGAGAACAGCAACC-3’ | |
| TLR4 1196C>T. F | 5’-CAAAGTGATTTCGGGACAAC-3’ | 284 |
| TLR4 1196C>T. F’ | 5’-CAAAGTGATTTCGGGACAAT-3’ | |
| TLR4 1196C>T. R | 5’-ACTTCGAGACTGGACAAGC-3’ | |
| HLA-DRB1.F | 5’-TGCCAAGTGGAGCACCCAA-3’ | 796 |
| HLA-DRB1.R | 5’-GCATCTTGCTCTGTGCAGAT-3’ |
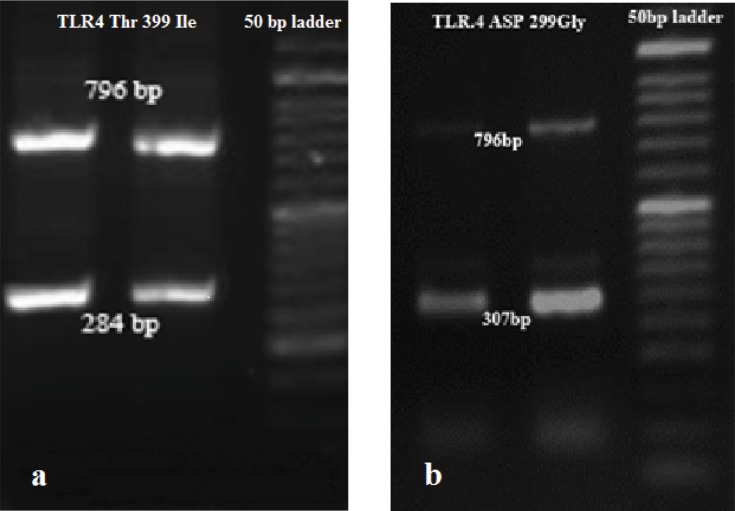
One cycle denaturation (95°C, 4’) followed by 20 cycles (95°C, 30’’; annealing at 65°C to 55°C, 5’’ for each degree; and elongation at 72°C for 20‘‘). The second step was repeated for 15 cycles (95°C, 10’’; 55°C, 30’’; 72°C, 20‘‘). (Bio-Rad Laboratories, Inc.). The third step included the final elongation at 72°C for 5 min. The amplified fragments of 896A>G and 1196C>T were electrophoresed on 1.5% agarose (CinnaGen, Iran) gel, stained with GelRed dye (Biotium, Inc.) and photographed (Figure 1A and B).
Figure 1.
SSP-PCR analysis of the (A) 1196C>T (284 bp) and (B) 896A>G (307 bp) variants in the T2DM patients. This person was heterozygous for both TLR4: 896A>G and 1196C>T.
Abbreviations: SSP-PCR, sequence-specific primer PCR; T2DM, Type 2 diabetes mellitus.
Statistical Analysis
Categorical data were presented as numbers and percentages and continuous variables as means ± SD. Intergroup comparisons were performed using the χ2-test for testing relationships between categorical variables. Based on “KS normality test” the results have been mentioned using; Mann–Whitney U-test for non-parametric and t-test for parametric variables. The related results are shown in each related table. Hardy–Weinberg equilibrium analyses were performed to compare observed and expected genotype frequencies using the chi-square test.
Odds ratios (ORs) were calculated and reported within the 95% confidence limits. P < 0.05 was considered as statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences version 15.0 (SPSS Inc., Chicago, IL, USA). Linkage disequilibrium and haplotype analysis were done using software program SNPStats (https://www.snpstats.net/start.htm).27 Logistic regression was carried out with adjustment for potential confounding covariates (age, sex, BMI) to obtain the odds ratio (OR) for risk of T2DM and clinicopathological aspects in both SNPs at 95% confidence intervals (CI).
Results
Our study included 100 diabetic patients and 100 control subjects. Baseline characteristics of the cases and controls are shown in Table 1. The frequency distribution of the TLR4 1196C>T and TLR4 896A>G were in accordance with Hardy–Weinberg equilibrium (P> 0.05). The comparison of genotypes and allele frequencies of the 896A>G and 1196C>T polymorphisms in case and control groups are shown in Table 3. No homozygous genotype was detected in our study for these two SNPs. Allele frequency and genotype distribution of both polymorphisms were not statistically significant between the case and control groups. No association was detected between these two SNPs and T2DM (P> 0.05).
Table 3.
Genotype and Allele Frequencies of 896A>G (Rs4986790) and 1196C>T (Rs4986791) of TLR4 Gene Among 100 Diabetes Mellitus Cases and 100 Controls
| 896A>G (rs4986790) |
Case (n=100) | Control (n=100) | χ2 | P | OR (95% CI) |
|---|---|---|---|---|---|
| Genotypes, n (%) | |||||
| AA | 81 (81%) | 88 (88%) | 1.87 | 0.17 | 0.78 (0.56–1.07) |
| AG | 19 (19%) | 12 (12%) | |||
| Alleles, n (%) | |||||
| A | 181(90%) | 188 (94%) | 1.71 | 0.19 | 0.8 (0.59–1.07) |
| G | 19 (10%) | 12 (6%) | |||
|
1196C>T (rs4986791) | |||||
| Genotypes, n (%) | |||||
| CC | 90 (90%) | 92 (92%) | 0.24 | 0.62 | 0.89 (0.57–1.38) |
| CT | 10 (10%) | 8 (8%) | |||
| Alleles, n (%) | |||||
| C | 190 (95%) | 192 (96%) | 0.23 | 0.62 | 0.89 (0.58–1.37) |
| T | 10 (5%) | 8 (4%) | |||
Notes: P < 0.05 was considered as statistically significant.
These two TLR4 polymorphisms were in strong linkage disequilibrium (D= 0.0091, D’= 0.2202, r= 0.164, P= 0.001). Haplotype analysis showed no association with T2DM in our study (global P = 0.86; Table 4).
Table 4.
Haplotype Association with Response (n = 200)
| Haplotype | Frequency | Case (n=100) | Control (n=100) |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| AC | 0.89 | 1.00 | 1.00 |
| GC | 0.06 | 1.20 (0.33–4.27) | 5.66 (1.49–21.53) |
| AT | 0.03 | 4.50 (0.70–29.00) | 2.38 (0.48–11.89) |
| GT | 0.01 | 0.41 (0.01–24.88) | 0.00 (-Inf - Inf) |
Note: Interaction p-value= 0.31; P < 0.05 was considered as statistically significant.
In 896A>G position, the levels of FBS (171.2/142.3) and OGTT (163.5/109.7) have shown to be significantly increased in heterozygous genotype (AG) compared with AA subjects (Table 5). Logistic regression showed the significant association between AG genotype and increased OGTT level (P= 0.048, OR= 1.003, 95% CI= (1.00–1.005)) (Supplementary Table 2).
Table 5.
Associations Between 896A>G Genotype and Clinicopathological Variables
| Total Population | AA (n) | AG (n) | P |
|---|---|---|---|
| BMI | 24 (22–26) (169) | 24 (22–29) (31) | 0.42 |
| FBS (mg/dL) | 109 (96 −184) (169) | 144 (105 −246) (31) | 0.04 |
| HDL (mg/dL) | 45 (37–56) (169) | 42 (39–46) (31) | 0.31 |
| LDL (mg/dL) | 89 (78–05) (169) | 98 (76 −109) (31) | 0.99 |
| TG (mg/dL) | 136.8 ± 73.5 (169) | 159 ± 64.1 (31) | 0.45 |
| Chol (mg/dL) | 180.4 ± 37.3 (169) | 179.8 ± 39.1 (31) | 0.98 |
| In cases only | |||
| HbA1C (%) | 4.1 ± 0.3 (81) | 5.8 ± 0.8 (19) | 0.06 |
| OGTT (mg/dl) | 109.8 ±132.5 (81) | 163.5 ± 155 (19) | 0.04 |
| T2DM duration (y) | 9.7 ± 5.3 (76) | 8.6 ± 5.4 (15) | 0.45 |
| Hypertension (n) | 31 | 6 | 0.58 |
| Family history IHD (n) | 28 | 9 | 0.29 |
| Statin therapy (n) | 41 | 8 | 0.63 |
| Nephropathy (n) | 10 | 1 | 0.37 |
Notes: Parametric values are presented as mean ± standard deviation and non-parametric data are presented as median (Q1-Q3). Data were evaluated using χ2-test for Hypertension, IHD, Statin therapy and Nephropathy; Mann–Whitney U-test for Age, BMI, HDL, LDL, TG and FBS; t-test for the other variables. P < 0.05 was considered as statistically significant.
Abbreviations: OGTT, oral glucose tolerance test; IHD, ischemic heart disease.
For 1196C>T; Table 6 shows the comparison of CC and CT genotypes with the clinicopathological aspects. After logistic regression (Supplementary Table 2), a significant association was shown between diabetic nephropathy (DN) and CT genotype (P= 0.04, OR= 4.35, CI= (1.04–18.1)). Also, TG level has increased significantly in both T2DM and control subjects with a heterozygous genotype of 1196C>T (P= 0.027, OR= 1.005, 95% CI= (1.001–1.01)).
Table 6.
Associations Between 1196C>T Genotype and Clinicopathological Variables
| Total Population | CC (n) | CT(n) | P |
|---|---|---|---|
| BMI | 24 (22–26) (182) | 24.7 (22.5 −27) (18) | 0.79 |
| Chol (mg/dL) | 174.5 (151–202) (182) | 187.5 (148–210) (18) | 0.99 |
| HDL (mg/dL) | 45 (38–56) (182) | 42.5 (37–49) (18) | 0.29 |
| LDL (mg/dL) | 92 (78−107) (182) | 91 (76–107) (18) | 0.65 |
| TG (mg/dL) | 120 (86–169) (182) | 150 (82–193) (18) | 0.32 |
| FBS (mg/dL) | 109 (96–185) (182) | 136 (98–237) (18) | 0.35 |
| In cases only | |||
| OGTT (mg/dl) | 116.5 ± 137.8 (90) | 134.1 ± 135.3 (10) | 0.86 |
| HbA1c (%) | 4.39 ± 4.6 (90) | 4.83 ± 4.5 (10) | 0.85 |
| T2DM duration (y) | 9 (6−12) (83) | 11 (7–15) (9) | 0.59 |
| Hypertension (n) | 30 | 7 | 0.02 |
| Family history IHD (n) | 33 | 4 | 0.83 |
| Statin therapy (n) | 45 | 4 | 0.75 |
| Nephropathy (n) | 8 | 3 | 0.04 |
Notes: Parametric values are presented as mean ± standard deviation and non-parametric data are presented as median (Q1-Q3). Data were evaluated using χ2-test for Hypertension, IHD, Statin therapy and Nephropathy; Mann–Whitney U-test for BMI, Chol, HDL, LDL, TG, FBS and T2DM duration (y); t-test for the other variables. P < 0.05 was considered as statistically significant.
Abbreviations: OGTT, oral glucose tolerance test; IHD, ischemic heart disease.
Discussion
To date, it is considered that the incidence of T2DM and its complication could be influenced by inflammation and immunity. Among inflammatory factors, TLR4 as an innate immune receptor can mediate inflammatory reactions.28 Several studies investigated the association between the TLR4 896A>G and 1196C>T polymorphisms and T2DM, but their results have been highly controversial.23 There are also limited studies on TLR4 in the inflammatory activation pathway and T2DM complications. In this regard, we focused on assessing TLR4 polymorphisms influence on T2DM incidence and its clinicopathological aspects in Iranian population.
This study failed to detect any homozygous variant genotypes of 896A>G and 1196C>T polymorphisms in Iranian subjects. Also, we could not find any association between these two polymorphisms and T2DM susceptibility in our population. Our finding is consistent with several studies in the Asian population, which showed a lack of association between 896A>G and 1196C>T and T2DM29,30 and is inconsistent with other investigations which showed that these polymorphisms were protective against T2DM especially in Caucasian populations.14,31,32 Interestingly in our investigation, all carriers of variant alleles of these two SNPs are heterozygous and based on the Erridge et al study, they could express a functional TLR4 molecule.33 However, other studies mentioned that the TLR4 pathway could be affected by other molecules such as MyD88 or NF-κβ in diabetes and insulin control.34,35
Previous investigations showed the metabolic syndrome role as a T2DM predictor.36 Regarding the impact of the TLR4 polymorphism on major features of the metabolic syndrome.37 The statistical association was assessed between variant genotype of 1196C>T and some clinicopathological features of T2DM such as TG, cholesterol, hypertension and BMI. Our findings are in line with Abbas et al study on rs5030717 and rs5030718 variants of TLR4 in the risk of dyslipidemia in type 2 diabetes mellitus38 and Kolz study,39 which found a strong interaction between total cholesterol to high-density lipoprotein cholesterol (TC/HDL-C) and minor alleles of four TLR4 variants (including 1196C>T alleles), using a case-cohort design. They suggested that TLR4 minor alleles of several variants might increase the risk for type 2 diabetes in subjects with high lipid profile via indirect effects.39 However, Illig et al29 did not find any difference in hypertension, BMI, waist circumference, or HDL/cholesterol levels in TLR4 896A>G variant.
Here, statistics showed the impact of 1196C>T variant on diabetic nephropathy (DN). Also, TG level has increased in subjects with heterozygous genotypes of 1196C>T in the present study. These results might be in accordance with the association between increased serum lipid and TLR4 activation,40 TLR4 polymorphisms involvement in the regulation of serum lipid metabolism41 and the role of disturbed lipid profiles, specifically TG in the pathogenesis of DN in different studies.42–47 Although, the small number of nephropathy (11%) should not rule out here. Further investigation could advantage the present data in a case–control study involving DN cases and diabetes as controls.
The TLRs expression in glomerular endothelial cells48 and TLR4 role in tubular inflammation is well documented.28 Lin et al showed a higher expression of TLR4 in the renal tubules of human kidneys with DN compared with normal kidney and other kidney diseases.28 Apparently, high glucose induces TLR4 expression in human proximal tubular epithelial cells and results in the release of proinflammatory mediators28 and leukocyte infiltration in the renal tissue.49 Consistent with our results, Abbas et al compared the frequencies of the TLR4 variants between nephropathy and dyslipidemia in T2DM patients, found a difference in the rs5030717 allele carriers.38 Kuwabara et al study in diabetic mouse models elucidated that hyperlipidemia has a pivotal role in the progression of DN through the activation of TLR4/S100 calcium-binding protein A8 signaling cascade in glomeruli.50
On the other hand, the mechanisms for higher triglyceride (TG) level in DN are affected by the duration of hyperglycemia and vascular endothelial damage, which in turn could be affected by TLR4 activation.47 This can also describe the association between AG genotype and increased OGTT level in this study. However, because of the limitations of our study, including the cross-sectional design which cannot establish causality, small sample size, and financial limitations, further investigations are recommended especially in DN patients compared with diabetics without nephropathy.
Conclusion
In conclusion, the results represented here suggest that minor alleles of 1196C>T and 896A>G variants, although not directly associated with type 2 diabetes, but by involving in the dysregulation of serum lipid levels and hyperglycemia, might increase the risk of DN. To our knowledge, the current report is the first study investigating the TLR4 variants in Iranian T2DM patients.
Acknowledgments
The authors are highly grateful to the Molecular Medicine Research Center for providing financial grant (No. 94162). This work was partially done in the Molecular Medicine Research Center, and in the Central Lab, School of Medicine, Hormozgan University of Medical Sciences, Bandar Abbas, Iran.
Disclosure
The authors declare that they had no conflict of interest.
References
- 1.Tillin T, Sattar N, Godsland IF, Hughes AD, Chaturvedi N, Forouhi NG. Ethnicity-specific obesity cut-points in the development of type 2 diabetes - a prospective study including three ethnic groups in the United Kingdom. Diabet Med. 2015;32(2):226–234. doi: 10.1111/dme.12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Abingdon). 2014;42(12):698–702. doi: 10.1016/j.mpmed.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Diabetes country profiles. World Health Organization; 2016. Available from: http://www.who.int/diabetes/country-profiles/irn_en.pdf?ua=1. Accessed March25, 2020.
- 4.Haghdoost AA, Rezazadeh-Kermani M, Sadghirad B, Baradaran HR. Prevalence of type 2 diabetes in the Islamic Republic of Iran: systematic review and meta-analysis. East Mediterr Health J. 2009;15(3):591–599. doi: 10.26719/2009.15.3.591 [DOI] [PubMed] [Google Scholar]
- 5.Habibzadeh F, Yadollahie M, Roshanipoor M, Haghighi AB. Prevalence of atrial fibrillation in a primary health care centre in Fars Province, Islamic Republic of Iran. East Mediterr Health J. 2004;10(1–2):147–151. [PubMed] [Google Scholar]
- 6.Habiba NM, Fulda KG, Basha R, et al. Correlation of lipid profile and risk of developing type 2 diabetes mellitus in 10–14 year old children. Cell Physiol Biochem. 2016;39(5):1695–1704. doi: 10.1159/000447870 [DOI] [PubMed] [Google Scholar]
- 7.Kalsi DS, Chopra J, Sood A. Association of lipid profile test values, type-2 diabetes mellitus, and periodontitis. Indian J Dent. 2015;6(2):81–84. doi: 10.4103/0975-962X.157270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haase CL, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R, Cholesterol HDL. Risk of type 2 diabetes: a mendelian randomization study. Diabetes. 2015;64(9):3328–3333. doi: 10.2337/db14-1603 [DOI] [PubMed] [Google Scholar]
- 9.Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol. 2003;41(7):1071–1077. doi: 10.1016/S0735-1097(03)00088-3 [DOI] [PubMed] [Google Scholar]
- 10.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327 [DOI] [PubMed] [Google Scholar]
- 11.Crook M. Type 2 diabetes mellitus: a disease of the innate immune system? An update. Diabet Med. 2004;21(3):203–207. doi: 10.1046/j.1464-5491.2003.01030.x [DOI] [PubMed] [Google Scholar]
- 12.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813–823. doi: 10.2337/diacare.27.3.813 [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Real JM, Pickup JC. Innate immunity, insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2008;19(1):10–16. doi: 10.1016/j.tem.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 14.Manolakis AC, Kapsoritakis AN, Tiaka EK, et al. TLR4 gene polymorphisms: evidence for protection against type 2 diabetes but not for diabetes-associated ischaemic heart disease. Eur J Endocrinol. 2011;165(2):261–267. doi: 10.1530/EJE-11-0280 [DOI] [PubMed] [Google Scholar]
- 15.Rudofsky G, Reismann P, Witte S, et al. Asp299Gly and Thr399Ile genotypes of the TLR4 gene are associated with a reduced prevalence of diabetic neuropathy in patients with type 2 diabetes. Diabetes Care. 2004;27(1):179–183. doi: 10.2337/diacare.27.1.179 [DOI] [PubMed] [Google Scholar]
- 16.Rallabhandi P, Bell J, Boukhvalova MS, et al. Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J Immunol. 2006;177(1):322–332. doi: 10.4049/jimmunol.177.1.322 [DOI] [PubMed] [Google Scholar]
- 17.Peterson JM, Mart R, Bond CE. Effect of obesity and exercise on the expression of the novel myokines, Myonectin and Fibronectin type III domain containing 5. PeerJ. 2014;2:e605. doi: 10.7717/peerj.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol. 2016;12(1):13–26. doi: 10.1038/nrneph.2015.175 [DOI] [PubMed] [Google Scholar]
- 19.Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J Clin Endocrinol Metab. 2014;99(1):39–48. doi: 10.1210/jc.2013-3092 [DOI] [PubMed] [Google Scholar]
- 20.Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587–591. doi: 10.2147/DMSO.S67400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fathya WM, Solimana MA, Raghebb A, Al Ashramc GH. Study of toll-like receptor 4 in type 2 diabetic patients with or without nephropathy. Menoufia Med J. 2016;29(1):167–173. doi: 10.4103/1110-2098.179009 [DOI] [Google Scholar]
- 22.Cai H, Cai J, Tao G. Association of toll-like receptor 4 polymorphisms with type 2 diabetes mellitus. APMIS. 2013;121(7):605–611. doi: 10.1111/apm.12027 [DOI] [PubMed] [Google Scholar]
- 23.Peng D, Wang J, Pan J, et al. Lack of association between TLR4 genetic polymorphisms and diabetic nephropathy in a Chinese population. Biomed Res Int. 2014;2014:704167. doi: 10.1155/2014/704167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin YW, Wang Q, Sun QQ, Hu AM, Liu HL. Toll-like receptor 4 gene Asp299Gly and Thr399Ile polymorphisms in type 2 diabetes mellitus: a meta-analysis of 15,059 subjects. Diabetes Res Clin Pract. 2015;107(3):338–347. doi: 10.1016/j.diabres.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S124–S138. doi: 10.2337/dc19-S011 [DOI] [PubMed] [Google Scholar]
- 26.Tajik N, Nasiri MR, Jafari M, et al. Association between toll-like receptor 4 (TLR4) genetic polymorphisms and susceptibility to pulmonary tuberculosis. Razi J Med Sci. 2010;16(68):19–26. [Google Scholar]
- 27.Sole X, Guino E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22(15):1928–1929. doi: 10.1093/bioinformatics/btl268 [DOI] [PubMed] [Google Scholar]
- 28.Lin M, Yiu WH, Wu HJ, et al. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. 2012;23(1):86–102. doi: 10.1681/ASN.2010111210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Illig T, Bongardt F, Schopfer A, et al. The endotoxin receptor TLR4 polymorphism is not associated with diabetes or components of the metabolic syndrome. Diabetes. 2003;52(11):2861–2864. doi: 10.2337/diabetes.52.11.2861 [DOI] [PubMed] [Google Scholar]
- 30.Liu F, Lu W, Qian Q, Qi W, Hu J, Feng B. Frequency of TLR 2, 4, and 9 gene polymorphisms in Chinese population and their susceptibility to type 2 diabetes and coronary artery disease. J Biomed Biotechnol. 2012;2012:373945. doi: 10.1155/2012/373945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagarolli RA, Saad MJ, Saad ST. Toll-like receptor 4 and inducible nitric oxide synthase gene polymorphisms are associated with type 2 diabetes. J Diabetes Complications. 2010;24(3):192–198. doi: 10.1016/j.jdiacomp.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 32.Kolek MJ, Carlquist JF, Muhlestein JB, et al. Toll-like receptor 4 gene Asp299Gly polymorphism is associated with reductions in vascular inflammation, angiographic coronary artery disease, and clinical diabetes. Am Heart J. 2004;148(6):1034–1040. doi: 10.1016/j.ahj.2004.05.049 [DOI] [PubMed] [Google Scholar]
- 33.Erridge C, Stewart J, Poxton IR. Monocytes heterozygous for the Asp299Gly and Thr399Ile mutations in the Toll-like receptor 4 gene show no deficit in lipopolysaccharide signalling. J Exp Med. 2003;197(12):1787–1791. doi: 10.1084/jem.20022078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orr JS, Puglisi MJ, Ellacott KL, Lumeng CN, Wasserman DH, Hasty AH. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes. 2012;61(11):2718–2727. doi: 10.2337/db11-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842(3):446–462. doi: 10.1016/j.bbadis.2013.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care. 2003;26(11):3153–3159. doi: 10.2337/diacare.26.11.3153 [DOI] [PubMed] [Google Scholar]
- 37.Mahdavi M, Fallah Z, Kelishadi R. Expression of Toll-Like receptors in metabolic syndrome: a systematic review. J Basic Res Med Sci. 2018;5(3):52–56. doi: 10.29252/jbrms.5.3.52 [DOI] [Google Scholar]
- 38.Abbas SA, Raza ST, Mir SS, et al. Role of variants rs5030717 and rs5030718 of TLR4 in the risk prediction of nephropathy, hypertension and dyslipidaemia in type 2 diabetes mellitus. Br J Biomed Sci. 2018;75(4):163–168. doi: 10.1080/09674845.2018.1477033 [DOI] [PubMed] [Google Scholar]
- 39.Kolz M, Baumert J, Muller M, et al. Association between variations in the TLR4 gene and incident type 2 diabetes is modified by the ratio of total cholesterol to HDL-cholesterol. BMC Med Genet. 2008;9(1):9. doi: 10.1186/1471-2350-9-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu YJ, Wang C, Song G, Zang SS, Liu YX, Li L. Toll-like receptor-2 and −4 are associated with hyperlipidemia. Mol Med Rep. 2015;12(6):8241–8246. doi: 10.3892/mmr.2015.4465 [DOI] [PubMed] [Google Scholar]
- 41.Qing YF, Zhou JG, Zhang QB, et al. Association of TLR4 Gene rs2149356 polymorphism with primary gouty arthritis in a case-control study. PLoS One. 2013;8(5):e64845. doi: 10.1371/journal.pone.0064845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stadler K, Goldberg IJ, Susztak K. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr Diab Rep. 2015;15(7):40. doi: 10.1007/s11892-015-0611-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutledge JC, Ng KF, Aung HH, Wilson DW. Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat Rev Nephrol. 2010;6(6):361–370. doi: 10.1038/nrneph.2010.59 [DOI] [PubMed] [Google Scholar]
- 44.Palazhy S, Viswanathan V. Lipid abnormalities in type 2 diabetes mellitus patients with overt nephropathy. Diabetes Metab J. 2017;41(2):128–134. doi: 10.4093/dmj.2017.41.2.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumsaiyai W. The impact of human adipose tissue on metabolic dysfunction in obesity and type 2 diabetes mellitus. Unpublished: Warwick Medical School; 2014.
- 46.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55(6):1832–1839. doi: 10.2337/db05-1620 [DOI] [PubMed] [Google Scholar]
- 47.Hirano T. Abnormal lipoprotein metabolism in diabetic nephropathy. Clin Exp Nephrol. 2014;18(2):206–209. doi: 10.1007/s10157-013-0880-y [DOI] [PubMed] [Google Scholar]
- 48.Anders HJ, Schlondorff D. Toll-like receptors: emerging concepts in kidney disease. Curr Opin Nephrol Hypertens. 2007;16(3):177–183. doi: 10.1097/MNH.0b013e32803fb767 [DOI] [PubMed] [Google Scholar]
- 49.Zhao H, Perez JS, Lu K, George AJ, Ma D. Role of Toll-like receptor-4 in renal graft ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;306(8):F801–F811. doi: 10.1152/ajprenal.00469.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuwabara T, Mori K, Mukoyama M, et al. Exacerbation of diabetic nephropathy by hyperlipidaemia is mediated by Toll-like receptor 4 in mice. Diabetologia. 2012;55(8):2256–2266. doi: 10.1007/s00125-012-2578-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- World Health Organization. Diabetes country profiles. World Health Organization; 2016. Available from: http://www.who.int/diabetes/country-profiles/irn_en.pdf?ua=1. Accessed March25, 2020.