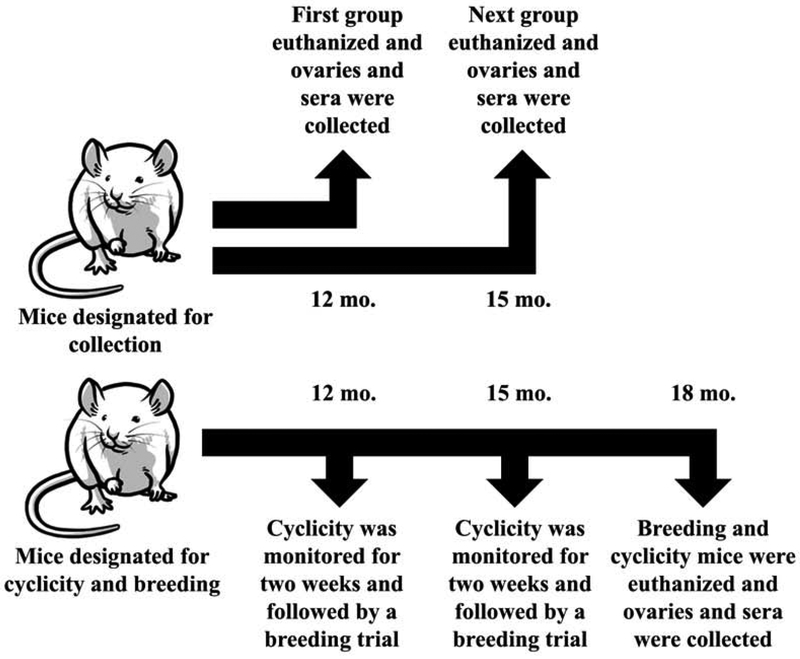
Figure 1.
Experimental Design. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). Following completion of dosing, mice were designated either for collection or for cyclicity and breeding. One group of mice designated for collection waited 12 months following completion of dosing before being euthanized for collection of ovaries and sera. An additional group of mice designated for collection waited 15 months following completion of dosing before being euthanized for collection of ovaries and sera. Mice that were designated for cyclicity and breeding waited 12 months following completion of dosing before they were monitored for cyclicity followed by a breeding trial. These same mice then waited an additional 3 months (totaling 15 months post-dosing) before they were again monitored for cyclicity and underwent another breeding trial. These same mice then waited an additional 3 months (totaling 18 months post-dosing) before they were euthanized for collection of ovaries and sera.