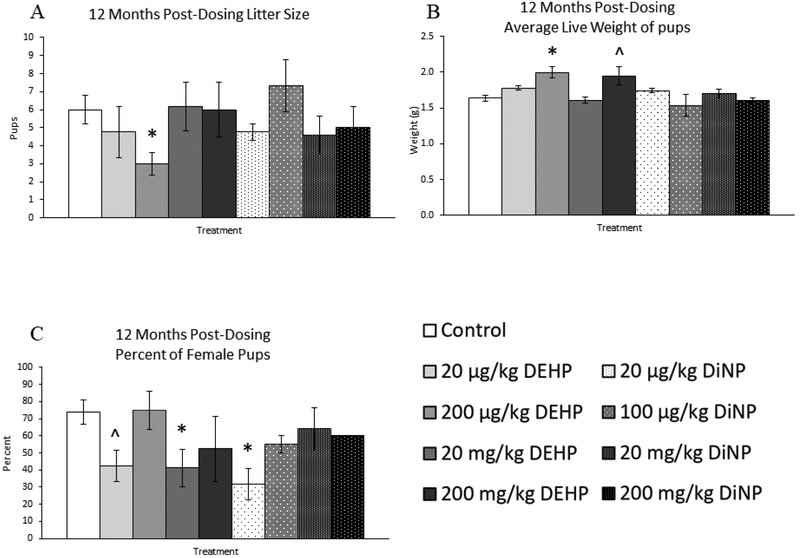
Figure 9.
Effects of DEHP and DiNP on litter outcomes at 12 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 12 months following completion of dosing, females were paired with untreated male CD-1 mice for breeding trials. If females delivered pups, litter size on PND 0 was determined (control n = 8 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 3–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 3–5 mice/group) (A), average weight of all live pups on PND 0 was measured (control n = 9 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 3–5 mice/group, DiNP 20 μg/kg/day – 20 mg/kg/day n = 4–5 mice/group, 200 mg/kg/day DiNP n = 2 mice) (B), and the percent of female pups was measured (control n = 7 mice/group, DEHP 20μg/kg/day – 20 mg/kg/day n = 4–5 mice/group, 200 mg/kg/day DEHP n = 2 mice, 20 μg/kg/day DiNP n = 3 mice, 100 μg/kg/day DiNP n = 2 mice, 20 mg/kg/day DiNP n = 5 mice, 200 mg/kg/day DiNP n = 1 mouse) (C). However, some groups lacked proper sample size to analyze outcomes, including average live weight for 200 mg/kg/day DiNP (n = 2 mice) and percent female pups for 200 mg/kg/day DEHP (n = 2 mice), 100 μg/kg/day DiNP (n = 2 mice), and 200 mg/kg/day DiNP (n=1 mouse). Statistical significance (p ≤ 0.05) is denoted with an asterisk (*). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).