Abstract
Exposure to trace metals may impact reproductive health outcomes through perturbations in maternal immune signaling molecules. We conducted a cross-sectional study of 390 pregnant women from the LIFECODES birth cohort and investigated the associations between 17 urinary metals and five immune biomarkers measured in the 3rd trimester (median 26 weeks gestation). We used linear regression to estimate pair-wise associations and applied elastic net and Bayesian kernel machine regression to identify important contributing exposures analytes as well as non-linear effects. Maternal urinary manganese, nickel, and barium were positively associated with maternal plasma interleukin-1β (IL-1β). Elastic net and Bayesian kernel machine regression identified manganese as the dominant trace metal in association with IL-1β. An interquartile range difference in manganese (0.6 μg/L) was associated with a 29% increase in IL-1β (95% CI: 12.4 – 48.2). In conclusion, trace metal exposures were associated with biomarkers of immune perturbations, and this warrants further investigation.
Keywords: trace metals, cytokines, immune perturbations
1. Introduction
The maternal immune system regulates numerous physiological processes to ensure the progression of a healthy pregnancy. Complex networks of immune signaling molecules guide placental development, protect the developing fetus from infection, facilitate communication between the fetal compartment and the maternal circulation, and maintain tolerance of paternal antigens [1–4]. Cytokines are proteins produced by immune cells and peripheral tissue and guide immune signaling by eliciting cellular responses such as chemotaxis, cell growth, differentiation, and apoptosis [2]. Historically, cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) have been grouped as pro-inflammatory markers, meanwhile interleukin-10 (IL-10) is known for its anti-inflammatory properties [2,5]. Additionally, C-reactive protein (CRP) is a non-specific inflammatory marker that is elevated in response to systemic inflammation [6]. Perturbations in these immunological biomarkers are associated with deleterious outcomes during pregnancy, including preterm birth and developmental effects on the fetus [2,7].
Within the context of human biology, essential trace metals are co-factors for physiological processes, (e.g., gene expression, enzymatic activity, cellular respiration), and this includes copper (Cu), manganese (Mn), nickel (Ni), selenium (Se), and zinc (Zn) [8]. In contrast, there are trace metals and metalloids that serve no physiological purpose, and are toxic to humans, including arsenic (As), barium (Ba), mercury (Hg), lead (Pb), thallium (Tl), beryllium (Be), cadmium (Cd), uranium (U), and tungsten (W). Overall, trace metals can interact with the maternal immune system through numerous pathways, including mitochondrial disruption, redox imbalance, interference of transcription factors, and acting as an adjuvant for innate and adaptive immune cells [9–14]. More specifically, one model of metal induced immune sensitization has indicated that cationic metal ions such as Ni2+ can induce dimerization of toll-like receptors in order to activate NF-κB signaling and up-regulate gene expression of cytokines [10]. Additionally, several metals can interfere with intracellular Ca2+ concentrations, which can directly influence the catalytic activity of Ca2+ dependent enzymes involved in immune signaling. For example, Pb exposure in animal models has been shown to inhibit Ca2+ dependent activity of protein kinases [15]. Ultimately, metals can interfere with several cellular signaling processes that can impact immune disruption.
Humans are exposed to mixtures of trace metals, which can occur through diet and contamination of soil, water, and air [16–27]. Regulations limited the use of some trace metals in products, such was the case with lead in paint and gasoline. However, anthropogenic factors such as industrialization, aging infrastructure, agriculture, and extraction of natural resources, contribute to the endurance of human exposure to trace metals in our environment [28]. Importantly, human studies have reported associations between trace metals and reproductive health outcomes such as preterm birth, and birth weight and size [29–35]. These studies underline the critical need to characterize associations with intermediate biomarkers of biological perturbations in order to disentangle toxicological mechanisms of trace metals during pregnancy.
To examine the pregnancy associations between trace metals and the maternal immune system, we performed a cross-sectional study of 17 urinary trace metals and 5 plasma immune biomarkers from samples collected at a single study visit (median 26 weeks gestation). We aimed to estimate single pollutant associations between individual trace metals and maternal immune biomarkers to inform potential mechanisms of adverse reproductive and developmental outcomes. Our secondary goal was to analyze the metals as a mixture to identify the most important predictors of outcome biomarker variables and. We further sought to evaluate cumulative exposures to mixtures of toxicants by constructing an environmental risk score for a select subset of metals. Very few studies have explored the relationships between mixtures of trace metals exposure and the maternal immune system during pregnancy [36–40]. Our study builds upon previous studies that focused on single pollutant analysis by evaluating multiple trace metals simultaneously [36–38,41]. One previous study analyzed an extensive panel of trace metals and utilized principal component analysis to analyze multiple metals simultaneously in association with biomarkers of inflammation [40]. Our study adds to the methodological approach of this previous study by leveraging two additional methods to analyze trace metal mixtures: elastic net combined with construction of an environmental risk score and Bayesian kernel machine regression. These methods offer additional insight into variable importance in the exposure predictor space, as well as estimates cumulative effects. We hypothesized that trace metal concentrations are associated with pro-inflammatory signals among plasma immunological biomarkers. We also hypothesized that cumulative metal concentrations would be associated with greater signals of inflammation.
2. Methods
2.1. Study population
Approximately 1,600 pregnant women were enrolled in the LIFECODES prospective birth cohort between 2006 and 2008 at the Brigham and Women’s Hospital in Boston, MA. Participants over 18 years of age were recruited early in pregnancy (<15 weeks gestation at the initial study visit). From the LIFECODES cohort, 1,181 participants were followed to term, and delivered live, single infants. Participants of this study attended up to four study visits throughout their pregnancy, and the range of each visit is as follows: visit 1 (4.71 – 16.1 weeks), visit 2 (14.9 – 21.2 weeks), visit 3 (22.9 – 29.3 weeks), and visit 4 (33.1 – 38.3 weeks). At the initial study visit, questionnaires were administered to collect demographic and health-related information. Urine and plasma samples were collected from each of the study visits. This study received institutional review board approval from the Brigham and Women’s Hospital.
We constructed an unmatched, nested case-control study from within this cohort, with additional details regarding recruitment found elsewhere [42,43]. The present analysis included participants from a pilot study for the Children’s Health and Exposure Assessment Resource (CHEAR) which was restricted to participants with urine samples available from visit 3. This resulted in a final sample size of 99 women who delivered preterm (< 37 weeks gestation) and 291 randomly selected women who delivered after 37 weeks gestation.
2.2. Measurement of urinary trace metals
Spot urine samples were stored at −80° C. A subset of 390 urine samples was available from study visit 3 and analyzed at NSF International for a panel of urinary trace metals (Ann Arbor, MI, USA). Methods to quantify urinary metal concentrations were developed based on the Centers for Disease Control and Prevention (CDC) Laboratory Procedure Manual for Urine Multi-Element Inductively Coupled Plasma-Dynamic Reaction Cell-Mass Spectrometry (ICP-DRC-MS) and have been previously described in detail (method no. 3018.3 and 3018A.2; revised 2012 March 19) [35]. We measured 17 trace metals using a Thermo Fisher (Waltham, MA, USA) iCAP RQ inductively coupled plasma mass spectrometer (ICPMS) with Teledyne CETAC Technologies (Omaha, NE, USA) ASX-520 autosampler. The non-essential trace metals that were measured included As, Ba, Be, Cd, Cr, Hg, Pb, Sn, Tl, U, and W. The essential trace metals included Cu, Mn, Mo, Ni, Se, and Zn. Metal concentrations were quantified from 1mL of urine. Urinary specific gravity was measured from 0.3mL of urine using a handheld digital refractometer (ATAGO Company Ltd., Tokyo, Japan).
Metal concentrations with no empirical value below the limit of detection (LOD) were assigned a value of , an imputation method that applies to exposure distributions that are not highly skewed and have at least 50 percent detection (Hornung and Reed 1990). Due to variations in urinary dilution, we corrected for urinary specific gravity in univariate and bivariate statistics, using the formula:
| [1] |
UBSG is the specific gravity-adjusted urinary biomarker concentration (μg/L), UB is the measured and uncorrected urinary biomarker concentration, the constant SGMedian (1.015) is the specific gravity population median, and SG is the observed specific gravity of the individual urine sample [44].
2.3. Measurement of immune biomarkers
Approximately 10mL of blood was drawn using ethylenediaminetetraacetic acid plasma tubes. Samples were temporarily stored at +4° C for less than 4 hours and then centrifuged for 20 minutes and stored at −80° C. Primary analyses focused on immune biomarkers measured in plasma samples collected at study visit 3 (n=346), and secondary analyses were conducted using plasma samples from study visit 4 (n=315). From plasma samples, we measured levels of endogenous immunological biomarkers, including the pro-inflammatory markers CRP, IL-1β, IL-6, and TNF-α and the anti-inflammatory marker IL-10. We selected these immune biomarkers based on several factors, including evidence of high detection in previous human studies and reported associations with adverse birth outcomes [45–48]. Quantification of immune biomarkers was performed at the Cancer Center Immunology Core at the University of Michigan (Ann Arbor, MI, USA). We used a DuoSet enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) to measure CRP. IL-1β, IL-6, TNF-α and IL-10 were simultaneously measured using the Milliplex MAP High Sensitivity Human Cytokine Magnetic Bead Panel (EMD Millipore Corp., St. Charles, MO). Samples below the lower LOD were imputed with LOD/√2, while samples above the upper LOD were imputed with the upper LOD. Further details on LOD values, detection rates, and assay sensitivity were previously described [49,50].
2.4. Data pre-processing for statistical analyses
Analyses were performed using R version 3.4.0. Due to the sampling approach for cases and controls, we designed inverse probability weights to account for over-representation of preterm birth cases in the nested case-control sample [51]. Briefly, we estimated the inverse probability of inclusion of cases and controls in the nested case-control sample relative to the overall proportions of cases and controls in the parent LIFECODES prospective birth cohort at Brigham and Women’s Hospital. We conducted weighted analyses for linear regression and for univariate and bivariate statistical analyses. The immunological biomarkers and highly detected urinary metals had distributions that were right-skewed and we transformed each of these variables using the natural log. We used the aikaike information criterion (AIC) to compare adjusted models with and without log-transformation. The following metals had very low detection rates, and were therefore modeled as binary variables (detect vs. non-detect) as: Be, Cd, Cr, U, and W.
2.5. Univariate and bivariate analyses
For descriptive statistics of urinary trace metals, we calculated geometric means (GM) and geometric standard deviations (GSD) following imputation of analytes below the LOD. The covariates that we reported in this study included: baseline maternal age, gestational age at sample collection, urinary specific gravity, baseline maternal education level (High school, technical school, some college, or college graduate), maternal race/ethnicity (White, African-American, or Other), maternal alcohol use and smoking during pregnancy (determined as yes/no), baseline health insurance provider – an indicator of socioeconomic status (Private/HMO/Self-pay vs. Medicaid/SSI/MassHealth), baseline maternal body mass index (BMI) at first study visit, and fetal sex (male/female). We tested for bivariate differences using simple linear regression for individual urinary trace metals regressed on each of the covariates individually. Pairwise spearman correlation coefficients were calculated for individual exposure analytes and immunological biomarkers. Interclass correlation coefficients (ICC) were calculated for immune biomarkers between the two available study visit measurements.
2.6. Multiple linear regression
We determined candidate confounders for adjusted analyses based on bivariate associations. Covariates were eligible for inclusion in regression models if they were associated with at least one exposure analyte and one inflammatory marker using linear regression. The variables that fit these criteria included: baseline maternal age, gestational age at sample collection, specific gravity, maternal education level, maternal race/ethnicity, maternal alcohol use and smoking during pregnancy, health insurance provider, BMI at initial study visit, and infant sex. From crude models, we included specific gravity and maternal age a priori, and then incorporated remaining variables in a stepwise manner that sequentially prioritized the variables with the most bivariate associations. Potential covariates were selected for the final model if they changed the beta coefficient of at least two trace metals by approximately 10%. We evaluated model fit and inspected the linearity of trace metals with respect to immunological biomarkers markers by using penalized splines within the GAM function of the mgcv package (version 1.8).
Primary adjusted linear regression models estimated associations between trace metals and immune biomarkers measured at study visit 3 (median 26 weeks). We then leveraged immune biomarker measurements from study visit 4 (median 35 weeks) and tested for associations with visit 3 trace metal measurements. Consistent associations with visit 4 measurements would provide evidence that our findings are more robust with respect to random chance, and that perhaps urinary trace metals may have latent effects on the maternal immune system. To enhance the interpretability of the regression coefficients of continuous outcome variables, we converted the beta coefficients of continuous exposure predictors and corresponding 95% confidence intervals to the percent change in inflammatory markers associated with an interquartile range difference in exposure analyte. The following equation was used to perform this conversion and applies only to the beta coefficients of continuous log-transformed exposure variables:
| [2] |
For the binary exposure variables in secondary analyses, we converted the beta coefficients to represent the percent change in inflammatory markers associated with detection of the exposure variable using the following equation:
| [3] |
We accounted for false positive associations and multiple statistical comparisons in our primary analyses by calculating q-values using the Bejamini and Hochberg method [52]. For these calculations, we treated each immune biomarker as a family of tests (12 tests with exposure analytes for each outcome biomarker). Associations with q-values below a threshold of 0.1 were interpreted with greater confidence, while associations above this threshold were recognized as greater risk of being a false positive.
2.8. Elastic net and environmental risk score
Our first goal for analyzing mixtures of trace metals was to identify the most predictive trace metals associated with an outcome biomarker. Furthermore, we coupled the construction of an environmental risk score to accomplish a secondary goal, which was to estimate a cumulative effect among the trace metals that the elastic net identified as most predictive. We sought to implement mixtures analyses conditional on the immune biomarker that had the greatest count of associations from single pollutant models. We implemented the elastic net regularization method using the package gcdnet (version 1.0.5) for all of the highly detected continuous trace metals in order to perform variable selection and identify the most predictive trace metals [53]. Equation 4 below represents the elastic net, which combines the least absolute shrinkage and selection operator (LASSO) with ridge regression:
| [4] |
For individual i (i = 1, …, N), Yi is the continuous natural log-transformed variable IL-1β, and the matrix of exposures j (j = 1, …, p). is a row vector of covariates for individual i, and is a row vector of exposures for individual i. In elastic net regularization, the λ1 tuning parameter shrinks beta estimates corresponding to non-predictive exposure variables to zero while the λ2 tuning parameter stabilizes solution paths when there is substantial collinearity among the covariates [53]. We estimated the λ1and λ2 tuning parameters based on 5-fold cross-validations optimized for minimal prediction errors. Candidate λ2 values ranged from 0 to 1 by increments of 0.01. Cross-validations produced λ2 = 0 as the optimal value, and we set λ2 = 0 as we optimized for λ1 based on least squares loss. The optimal λ1 was 0.08.
The coefficients estimated by elastic net were then extracted and used as metal-specific weights. We weighted each individual participant’s measured metal concentration by these metal-specific weights, and then calculated the linear combination of all metals to construct a cumulative environmental risk score [54], represented here:
| [5] |
Here, represents the coefficient extracted from elastic net for predictor j weighted against each individual’s observed concentration of predictor j. Upon constructing the ERS, we regress a given outcome biomarker by the ERS and estimate the cumulative effect of the metals used to construct the ERS.
2.9. Bayesian kernel machine regression
In addition to identifying the most predictive trace metals, another goal that we pursued was evaluating non-linearity and potential interactions between trace metals. Our application of elastic net only models linear terms, therefore to complement the environmental risk score construction, we implemented Bayesian kernel machine regression [55] using the package bkmr (version 0.2.0), and represent the modeling framework here:
| [6] |
The function h(Xi), hereby called the exposure-response function, is a flexible kernel function of the exposure variables that can account for non-linear and non-additive relationships between the mixture of metals and IL-1β, while adjusting for a covariate matrix . Moreover, Bayesian kernel machine regression can perform variable selection by placing a spike-and-slab hyper-prior on the components of the kernel matrix corresponding to the prior variance on h(Xi). In our implementation of the Bayesian kernel machine regression, we set the kernel distribution to Gaussian, selected flat priors for parameter estimation, and iterated 10,000 times for the Markov chain Monte Carlo. We utilized the variable selection option only to estimate posterior inclusion probabilities as an indicator of variable importance to compare and contrast overall findings with the elastic net and the single pollutant models.
2.10. Sensitivity analyses
Previous studies have reported sex-specific differences in trace metals exposures [56] and associations with birth outcomes and development [57,58]. Therefore we wanted to determine if there were sex-specific associations with immune biomarkers in our study that could potentially inform sex differences observed in pregnancy outcomes. To accomplish this goal, we tested statistical interaction terms between exposure analytes and infant sex to determine sex differences in effect estimates. Additionally, we conducted a sensitivity analysis to test for differences in effect estimates by case status, where we tested statistical interaction terms between exposure analytes and preterm birth status in unweighted models.
3. Results
3.1. Descriptive statistics
In Table 1 we report the raw and weighted characteristics of participants from the LIFECODES cohort who were included in the present analysis. A majority of the study participants were over the age of 30 (71.4%), White (59.7%), obtained higher education (86.7%), and were privately insured (82.7%). Approximately 55% of the participants had a male fetus. Smoking and drinking during pregnancy occurred in 6% and 4.7% of participants, respectively. The detection rates of the inflammatory biomarkers were above 75%. We observed high detection (>75%) of 12 metals (As, Ba, Cu, Hg, Mn, Mo, Ni, Pb, Se, Sn, Tl, and Zn) in urine samples (Table 2). Meanwhile, Be, Cd, Cr, U, and W all had detection rates below 50% (Table 2). We observed comparable concentrations in most of the urinary trace metals when compared to nationally representative surveys such as the 2007–2012 National Health and Nutrition Examination Survey (NHANES) and the 2007–2009 Canadian Health Measures Survey (CHMS) [35]. We did observe higher concentrations of As (LIFECODES GM = 18.4 μg/L; NHANES GM = 7.14 μg/L), Mn (LIFECODES GM = 0.8 μg/L, NHANES GM = 0.13 μg/L), and Ni (LIFECODES GM = 2.6 μg/L; NHANES GM = 1.0 μg/L) [35]. Among the highly detected (>75%) metals, spearman correlation coefficients ranged between metals ranged from −0.9 to 0.35, with the highest positive correlation observed between the toxic metals Ba and Ni (Spearman ρ = 0.35, p < 0.01) (Supplemental Figure 1). Correlations between metals and immune biomarkers were more modest, with the highest observed between Mn and IL-1β (Spearman ρ = 0.14, p < 0.01) (Supplemental Figure 1). ICC values (95%CI) for immune biomarkers ranged from moderate to high: CRP [0.62 (0.54, 0.68)], IL-1β [0.95 (0.94, 0.96)], IL-6 [0.99 (0.98, 0.99)], IL-10 [0.99 (0.99, 0.99)], TNF-α [0.93 (0.91, 0.94)] (Supplemental Table 1).
Table 1.
LIFECODES Prospective pregnancy cohort profile: raw counts and weighteda proportions (N=390)
| Population Characteristics | Raw Count (percent) | Weighted Count (percent) | |
|---|---|---|---|
| Age | 18 – 24 | 41 (10.5%) | 44 (11.2%) |
| 25 – 29 | 76 (19.5%) | 78 (19.9%) | |
| 30 – 34 | 159 (40.8%) | 157 (40.3%) | |
| 35 + | 114 (29.2%) | 112 (28.6%) | |
| Race/ethnicity | White | 234 (60.0%) | 233 (59.7%) |
| African-American | 58 (14.9%) | 59 (15%) | |
| Other | 98 (25.1%) | 99 (25.3%) | |
| Education | High school degree (13 years) | 53 (13.6%) | 50 (12.9%) |
| Technical school (>13 years) | 59 (15.1%) | 5.7 (14.7%) | |
| Junior college or some college (>13 years) | 116 (29.7%) | 117 (30%) | |
| College graduate (16+ years) | 152 (39.0%) | 15 (39.5%) | |
| Missing | 10 (2.56%) | 11 (2.9%) | |
| Health Insurance Provider | Private/HMO/Self-pay | 317 (81.3%) | 314 (80.6%) |
| Medicaid/SSI/MassHealth | 64 (16.4%) | 66 (16.8%) | |
| Missing | 9 (2.31%) | 10 (2.6%) | |
| BMI at Initial Visit | <25 kg/m2 | 208 (53.3%) | 210 (53.9%) |
| 25 – 29.9 kg/m2 | 101 (25.9%) | 104 (26.6%) | |
| ≥ 30 kg/m2 | 79 (20.3%) | 74 (18.9%) | |
| Missing | 2 (0.51%) | 2 (0.6%) | |
| Tobacco Use | No smoking during pregnancy | 360 (92.3%) | 361 (92.6%) |
| Smoked during pregnancy | 25 (6.41%) | 23 (5.9%) | |
| Missing | 5 (1.28%) | 6 (1.5%) | |
| Alcohol Use | No alcohol use during pregnancy | 366 (93.8%) | 364 (93.3%) |
| Alcohol use during pregnancy | 15 (3.85%) | 18 (4.6%) | |
| Missing | 9 (2.31%) | 8 (2.2%) | |
| Fetal Sex | Female | 170 (43.6%) | 175 (45%) |
| Male | 220 (56.4%) | 215 (55%) | |
Weighted using inverse probability weighting to account for the sampling approach of the nested case-control sample
Table 2.
Weighteda distribution of specific gravity corrected urinary metals concentrations (μg/L) (N=390 samples)
| Select Percentiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Analyte (μg/L) | LOD | % Above LOD | GM | GSD | 25th | 50th | 75th | 90th | 95th |
| As | 0.3 | 100% | 18.4 | 2.7 | 9.6 | 17.8 | 32.4 | 67.2 | 97.6 |
| Ba | 0.1 | 99% | 1.7 | 2.8 | 1.0 | 2.0 | 3.4 | 5.2 | 6.2 |
| Be | 0.04 | 8% | 0.02 | 2.8 | <LOD | <LOD | <LOD | <LOD | 0.05 |
| Cd | 0.06 | 44% | 0.1 | 3.0 | <LOD | 0.1 | 0.1 | 0.3 | 0.5 |
| Cr | 0.4 | 15% | 0.2 | 3.1 | <LOD | <LOD | <LOD | 0.7 | 1.1 |
| Cu | 2.5 | 92% | 9.4 | 1.7 | 7.0 | 9.1 | 12.1 | 15.4 | 19.6 |
| Hg | 0.05 | 92% | 0.5 | 2.9 | 0.3 | 0.5 | 1.0 | 1.5 | 2.0 |
| Mn | 0.08 | 98% | 0.8 | 2.4 | 0.5 | 0.7 | 1.1 | 2.4 | 5.5 |
| Mo | 0.3 | 100% | 51.7 | 1.8 | 37.2 | 51.3 | 69.3 | 100.8 | 127.6 |
| Ni | 0.8 | 86% | 2.6 | 2.0 | 1.9 | 2.8 | 4.0 | 5.5 | 6.8 |
| Pb | 0.1 | 76% | 0.3 | 3.9 | 0.2 | 0.4 | 0.6 | 1.0 | 1.3 |
| Se | 5.0 | 99% | 37.4 | 1.4 | 30.2 | 37.2 | 45.9 | 56.9 | 66.4 |
| Sn | 0.1 | 94% | 0.7 | 2.9 | 0.4 | 0.6 | 1.2 | 2.7 | 4.9 |
| Tl | 0.02 | 84% | 0.1 | 2.5 | 0.1 | 0.1 | 0.2 | 0.2 | 0.3 |
| U | 0.01 | 12% | 0.0 | 3.1 | <LOD | <LOD | <LOD | 0.01 | 0.02 |
| W | 0.2 | 21% | 0.1 | 2.6 | <LOD | <LOD | <LOD | 0.4 | 0.5 |
| Zn | 2.0 | 100% | 226.9 | 2.1 | 148 | 246 | 366 | 542 | 653 |
Abbreviations: LOD (Limit of detection), GM (geometric mean), GSD (geometric standard deviation), As (Arsenic), Ba (Barium),
Be (Beryllium), Cd (Cadmium), Cr (Chromium), Cu (Copper), Hg (Total mercury), Mn (Manganese), Mo (Molybdenum), Ni (Nickel),
Pb (Lead), Se (Selenium), Sn (Tin), Tl (Thallium), U (Uranium), W (Tungsten), Zn (Zinc).
Weighted using inverse probability weighting to account for the sampling approach of the nested case-control sample
3.2. Regression results
The final group of variables used in multivariable linear regression included individual continuous natural log-transformed inflammatory markers regressed on natural log-transformed exposure analytes, specific gravity, maternal age, race/ethnicity, and BMI at initial visit. Based on smoothing plots and the AIC between models with and without penalized splines, we found that splines for exposures did not significantly improve the model fit. In Figures 1A and 1B we present the percent change in inflammatory biomarkers (measured in visits 3 and 4 plasma) associated with an interquartile range difference in continuous trace metals (measured in visit 3 urine) from adjusted multiple linear regression models.
Figure 1.
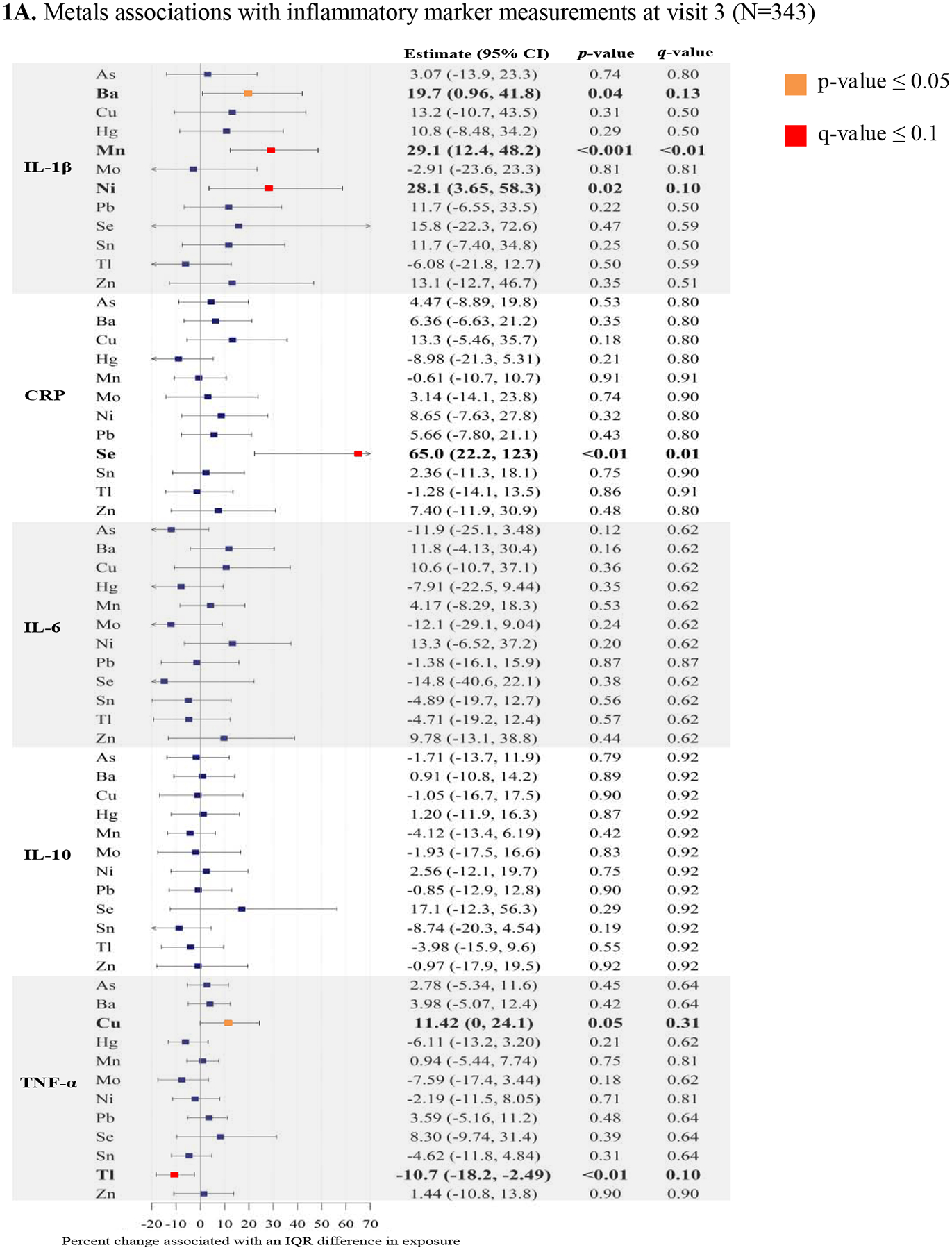
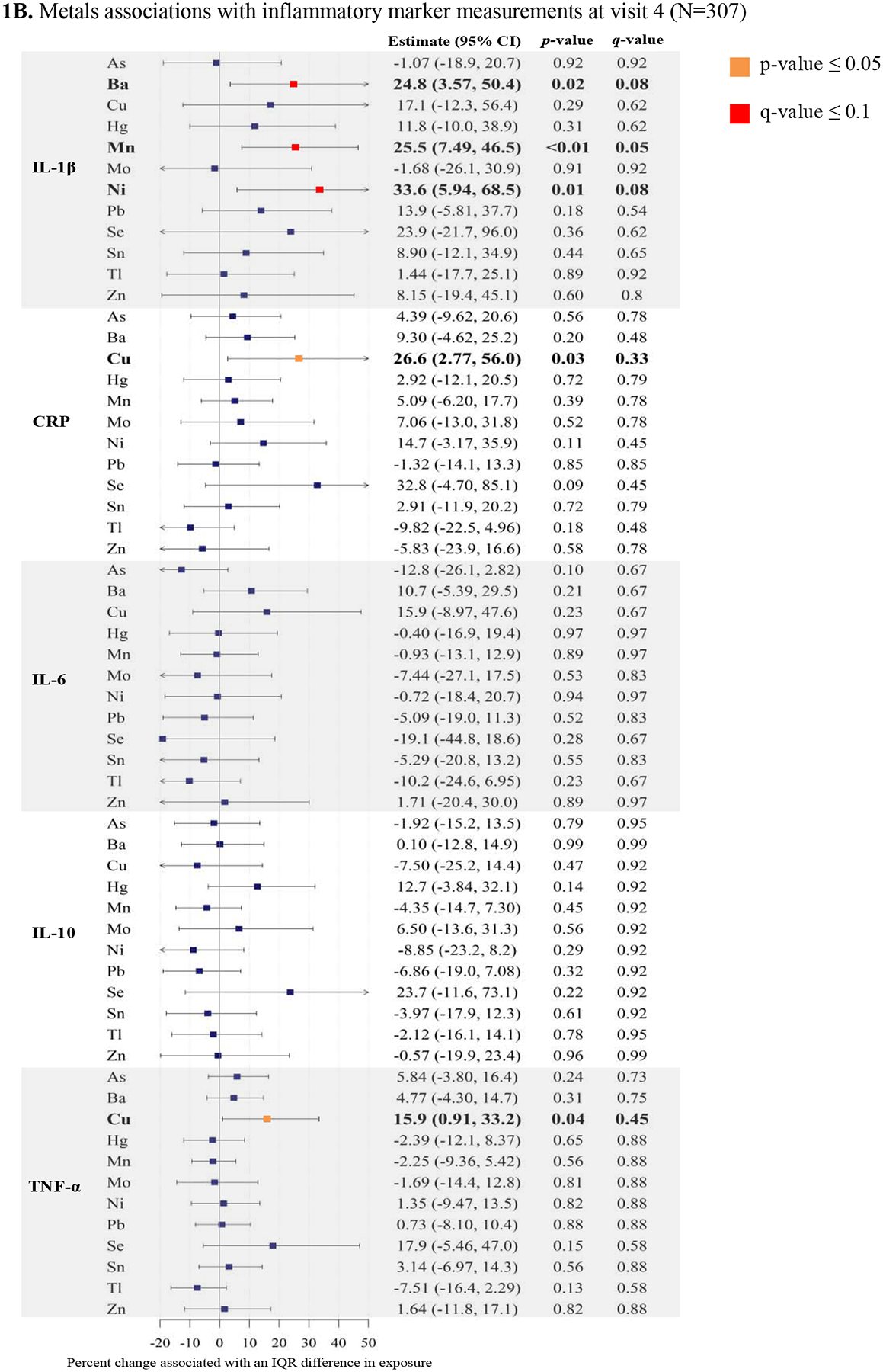
Forest plots of adjusted linear regressiona analyses: Percent change in immune biomarkers associated with an interquartile range (IQR)b increase in urinary trace metals with corresponding p-values, and q-values.
Abbreviations: As (Arsenic), Ba (Barium), Cu (Copper), Hg (Total mercury), Mn (Manganese), Mo (Molybdenum), Ni (Nickel), Pb (Lead), Se (Selenium), Sn (Tin), Tl (Thallium), Zn (Zinc), CRP (C-reactive protein), IL-1β (Interleukin-1β), IL-6 (Interleukin-6), IL-10 (Interleukin-10), TNF-α (Tumor necrosis factor- α).
aLinear regression models adjusted for specific gravity, maternal age, maternal race/ethnicity, and body mass index at initial study visit
bIQR calculated from uncorrected, untransformed, weighted distributions of urinary trace metals
We observed that three metals were positively associated with the pro-inflammatory cytokine IL-1β. Among the essential trace metals, an interquartile range in Mn (0.6 μg/L) was associated with a 29.1% increase in IL-1β (95% CI: 12.4, 48.2), and an interquartile range difference in Ni (2.8 μg/L) was associated with a 28.1% increase in IL-1β (95% CI: 3.65, 58.3) (Figure 1A). An interquartile range difference in the toxic trace metal Ba (2.2 μg/L), was associated with a 19.7% increase in IL-1β (95% CI: 0.96, 41.8) (Figure 1A). In relation to another pro-inflammatory cytokine, TNF-α, an interquartile range difference in Cu (9.0 μg/L) was associated with an 11.4% increase in TNF- α (95% CI: 0, 24.1) (Figure 1A). In contrast, we observed that an interquartile range difference in Tl (0.1 μg/L) was associated with a 10.7% decrease in TNF-α (95% CI: −18.2, −2.49) (Figure 1A). The essential trace element Se was associated with the general pro-inflammatory biomarker C-reactive protein, and an interquartile range difference in Se (38.9 μg/L) was associated with a 65% increase in C-reactive protein (95% CI: 22.2, 123) (Figure 1A). We did not observe notable associations with the cytokines IL-6 or IL-10. Among the associations with p < 0.05, the discussed associations with Mn, Ni, and Se all had q-values < 0.1, providing greater confidence that these associations may not be false discoveries (Figure 1A).
Among the metals with low detection rates, we observed that the detection of U was associated with a 105% increase in IL-1β (95% CI: 37.6, 206) (Supplemental Table 2). Additionally, W detection was associated with 29.4% decrease in IL-10 (95% CI: −44.6, −10.1), and Cr detection was associated with a 32.1% increase in TNF-α (95% CI: 10.7, 57.7) (Supplemental Table 2).
Regression analyses between visit 3 trace metal measurements and visit 4 immune biomarkers are presented in Figure 1B and Supplemental Table 3. We observed consistent direction of association for the trace metals that were associated with IL-β (Ba, Mn, and Ni). Interestingly, the relationship between Cu and CRP became more associated with CRP measurements from visit 4. An IQR difference in Cu (9 μg/L) was associated with CRP (26.6% increase, 95% CI: 2.77, 56.0) (Figure 1B).
3.3. Mixtures analyses
IL-1β was the immune biomarker that had the greatest number of associations with trace metals, where positive associations were observed with Ba, Mn, and Ni. Elastic net selected and estimated coefficients for Mn (β = 0.12) and Ni (β = 0.03) while shrinking all other metals to zero (Supplemental Table 4). When we constructed the environmental risk score and regressed IL-1β by this risk score, we observed that an interquartile range difference in the environmental risk score (0.2 units) was associated with a 40.5% increase in IL-1β (95% CI: 20.8, 63.4), and this finding was also robust to false discovery (q < 0.001). Bayesian kernel machine regression indicated that among a mixture of three trace metals (Mn, Ni, and Ba), Mn exhibited the greatest effect estimate within the exposure-response function h(z) in association with IL-1β (Figure 2). There was a positive association between the cumulative exposure-response function of all metals, and the effect was significantly greater than zero when all metals were at the 70th percentile (Supplemental Figure 2). Posterior inclusion probabilities estimated by Bayesian kernel machine regression agreed with variable selection estimated by elastic net: Mn (0.98), Ni (0.25), and Ba (0.16) (Supplemental Table 5). While holding all other metals constant at various quantiles, Mn’s contribution to the exposure-response function h(z) exhibited a curvilinear and slightly u-shaped association with IL-1β, where lower Mn concentrations had a negative effect estimate and higher Mn concentrations had a positive effect estimate (Figure 2). Further, the association between Mn and IL-1β did not appreciably differ by varying quantiles of Ba or Ni (Figure 2). Ni exhibited a non-linear association with IL-1β in the exposure-response function, however the confidence intervals of this fitted function consistently overlapped with the null effect threshold of zero (Supplemental Figure 3). In the exposure-response function, the association between Ba and IL-1β appeared null, with some suggestive evidence for effect modification at the 90th percentile of Mn (Figure 2). Taken together, these approaches for examining mixtures identified Mn as a dominant predictor of IL-1β.
Figure 2.
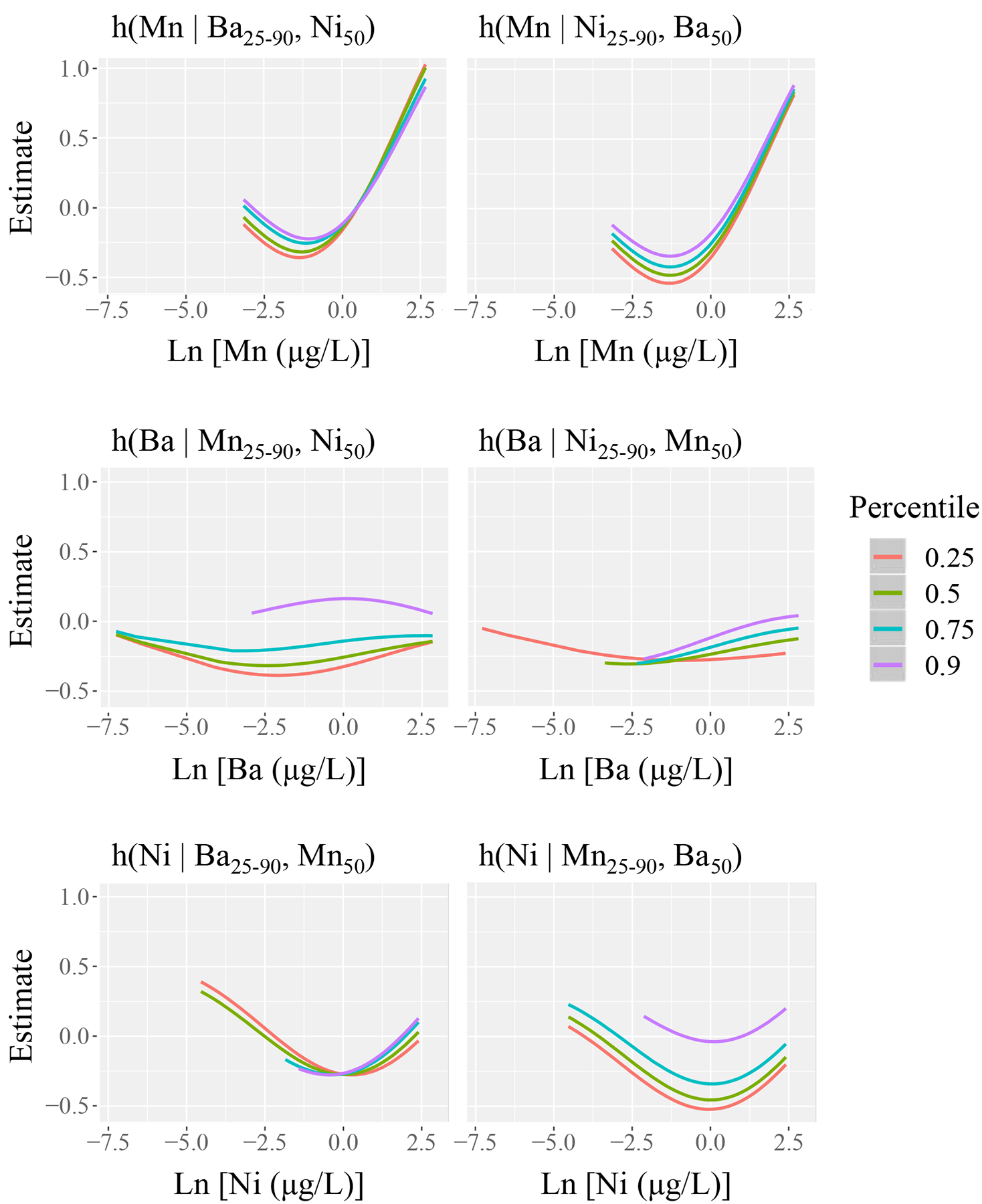
Estimation of the exposure-response function h(z) using Bayesian kernel machine regression: the relationship between interleukin-1β (IL-1β) and barium (Ba), manganese (Mn), and nickel (Ni). Each graphical unit reports h(z) as a function of one trace metal, conditional on four different quantiles of a second trace metal, while holding the third trace metal constant at the 50th percentile.
3.4. Sensitivity analyses
Among the tests for interactions with fetal sex, most of the associations did not vary by fetal sex (pinteraction > 0.05). However, in the association between Mn and IL-1β, we observed evidence for statistical interaction with fetal sex (pinteraction = 0.01), such that women with a female fetus had higher positive associations than women with a male fetus (Figure 3). We also present fetal sex-stratified regression results between trace metals and IL-1β in Supplemental Table 6. An IQR difference in Mn (0.67 μg/L) among women with a female fetus was associated with a 52.2% increase in IL-1β (95% CI: 25.3, 84.9) (Supplemental Table 6). Meanwhile an IQR difference in Mn (0.60 μg/L) among women with a male fetus was associated with a 2.56% increase in IL-1β (95% CI: −15.1, 23.9) (Supplemental Table 6). Although tests for interactions did not find differences by fetal sex for Ba and Ni, the stratified analysis showed that the positive effects of Ba and Ni on IL-1β was observed in women with a female fetus but not in women with a male fetus. For women with a female fetus, an IQR difference in Ba (2.25 μg/L) was associated with a 53.1% increase in IL-1β (95% CI: 13.0, 107), and an IQR difference in Ni (2.82 μg/L) was associated with a 56.5% increase in IL-1β (95% CI: 7.13, 128) (Supplemental Table 6). When we tested for statistical interactions between trace metals and preterm birth status across all inflammatory markers, we observed no evidence for interactions (p > 0.05). Regression analysis among only the controls showed consistent associations compared to the weighted analysis of overall study sample for: Se and CRP; Mn and IL-1β; U and IL-1β; W and IL-10; Tl and TNF-α; and W and TNF-α (Supplemental Table 7).
Figure 3.
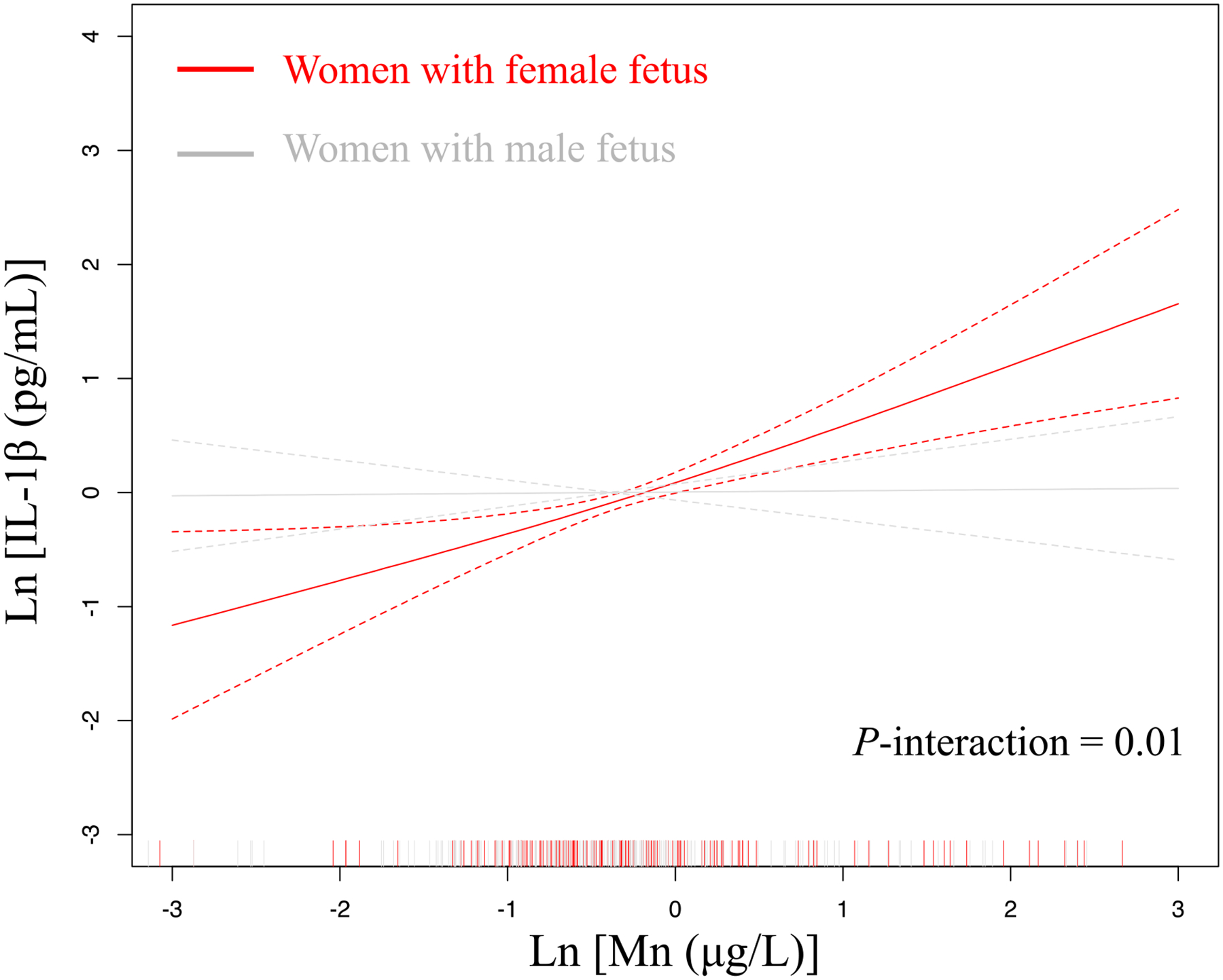
Adjusted associations between manganese (Mn) and interleukin-1β (IL-1β) stratified by fetal sex
4. Discussion
In this study we investigated the associations between urinary biomarkers of environmental trace metals exposures and plasma biomarkers of the maternal immune system. Notably, this study demonstrated that urinary concentrations of Mn, Ba, Ni, Se, and Cu are associated with increased biomarker signals of inflammation within the maternal immune system, while Tl was the only trace metal that exhibited an anti-inflammatory association. Detection of U, Cr, and W were also associated with altered maternal cytokine concentrations. Additionally, we explored two approaches to characterize mixtures of trace metals in association with IL-1β: elastic net and Bayesian kernel machine regression. Elastic net identified Mn and Ni as most predictive of IL-1β and their cumulative effect was larger than the individual coefficients. Bayesian kernel machine regression reported consistent findings, where Mn was the dominant essential metal contributing to the highest effect estimates within the exposure-response function, followed by Ni. We also observed mostly consistent associations when comparing findings for immune biomarkers measured in visit 3 and 4. The associations that differed between visits could be due in part to varying sample sizes across study visits given that some women delivered by study visit 4, or by the temporal difference in measurement of immune biomarkers. Altogether, this study provides evidence that the maternal immune system may be a target of select trace metal exposures in pregnancy and can inform potential underlying mechanisms to explain adverse reproductive and developmental outcomes.
To our knowledge, this is the largest study to explore the associations between this panel of 17 urinary trace metals and maternal immunological biomarkers during pregnancy. Very few studies have been conducted on trace metals and circulating maternal cytokines during gestation [36–40]. One study of 56 women in Michigan measured trace metals in urine and cytokines in blood from samples collected between 8 and 14 weeks of gestation [40]. In this study, Kelley and colleagues [39] found that maternal exposure to Se, Mo, Ni, and Zn were positively associated with the chemotactic cytokine interleukin-8. Similar to our study, there were no associations between cytokines and the toxic metals Pb (GM = 0.22 μg/L compared to LIFECODES GM = 0.3 μg/L) or Hg (GM = 0.07 μg/L compared to LIFECODES GM = 0.5 μg/L). These concentrations are comparable or lower than previously published NHANES estimates: Hg (GM = 0.43 μg/L) and Pb (GM = 0.44 μg/L) [35]. These comparisons with NHANES suggest that perhaps the exposure levels we observed for these toxic metals may be low enough to not observe effects with immune biomarkers. Although we did not observe notable associations with As concentrations in urine, a previous study (n = 50 mother-newborn pairs) in the Biomarkers of Exposure to Arsenic cohort found evidence that maternal urinary As was associated with increased cord blood proteins involved in inflammation [36]. Additionally, in a sample of 61 mother-infant pairs from the Brazilian Amazon, Nyland and colleagues [35] tested for associations between blood Hg concentrations and immune biomarkers and did not find evidence for associations between maternal blood Hg and the cytokines IL-1β, IL-1ra, IL-6, IL-10, or TNF-α in maternal blood or cord blood. However, the study did find that maternal Hg was associated with increased total immunoglobulin-G (IgG), a marker of immunotoxicity [39]. These studies in combination with our findings underline the nuances of biological media for investigating immune disruption, and emphasize the need to measure exposures and endogenous biomarkers in multiple media in order to better understand the scope of toxic effects.
In our study, Ba, Mn, and Ni were all positively associated with IL-1β, and both elastic net and Bayesian kernel machine regression provided evidence that among all three of the metals, Mn had the largest effect estimate. Bayesian kernel machine regression also revealed a curvilinear u-shaped relationship between Mn and IL-β. Furthermore, when we implemented the elastic net to construct an environmental risk score of the linear combination of Mn and Ni, we found that the risk score was associated with increased effect estimates for IL-1β compared to the individual metals alone. Kelley and colleagues observed higher geometric average concentration of urinary Mn (1.44 μg/L) in the Michigan based cohort compared to our current study (0.8 μg/L) and did not observe an association between Mn and IL-1β [40]. However, the Michigan based study had a smaller sample size (n=56) and measured metal concentrations 8 – 14 weeks gestation while our study measured metals 22.9 – 29.3 weeks gestation. Interestingly, both our study and the Michigan based cohort observed higher Mn concentrations than previously reported in NHANES (GM = 0.13 μg/L) [35], indicating that the association we observed in our study may be partly driven by both the timing of exposure and higher average exposure level compared to a nationally representative sample. Although not directly comparable to our study sample, a larger sample of adults (N = 633) in the Normative Aging Study reported that dietary intake estimates of Mn was associated with increased IL-1β [59]. Additional studies are needed to replicate our findings with comparable sample characteristics and timing of exposure.
In our tests for statistical interactions, we found that the association between Mn and IL-1β was further contextualized by fetal sex, such that women with a female fetus showed positive associations between Mn and IL-1β, whereas women with a male fetus showed non-significant associations. Maternal cytokine profiles have previously been observed to differ based on fetal sex, underlining the potential for fetal sex to elicit unique maternal immune responses throughout pregnancy [60]. It is unclear whether the fetal sex-specific association we observed with Mn and IL-1β could be indicative of vulnerability for women with a female fetus, or if the association is a result of sex-specific maternal immune responses proceeding Mn exposure. Manganese metabolism has been shown to differ by sex, which is hypothesized of being dependent on iron concentrations [61]. Furthermore, fetal antioxidant responses to xenobiotics and drugs has been observably higher in females [62]. One potential hypothesis for our sex-specific findings could perhaps be due to a differential oxidative stress response to manganese exposure by female fetuses, which in turn may elicit a differential maternal immune response from women with a female fetus. Overall, the association we observed between Mn and IL-1β needs to be tested in additional prospective birth cohorts.
IL-1β is a pro-inflammatory cytokine predominantly produced by macrophages, and cleaved into its active form by inflammasomes, which are a cytosolic multi-protein complexes [63,64]. Secretion of IL-1β occurs in many ways, including direct cytosolic release via exocytosis and transportation within exosomes and vesicles [63,65]. Each of these secretion mechanisms are driven by many factors, including stimulus from pathogen-associated molecular patterns (PAMPs), danger associated molecular patterns (DAMPs), and redox balance within cells [63,65]. There are many possible pathways by which Mn and Ni can affect the expression and secretion of IL-1β. For example, Ni can elicit adjuvant properties through functional mimicry of PAMPs [10]. Mechanistic studies of Mn indicate that upon uptake in cells, Mn can be sequestered in mitochondria, resulting in disruption of Ca2+ transport, mitochondrial dysfunction, and intracellular redox imbalance [66]. Furthermore, Mn and Ni both have the capacity to interact with the transcription factor NF-κB, a potent regulator of gene expression for various cytokines and immune signaling peptides [10,66,67]. Cellular damage caused by Mn and Ni can also lead to the increased accumulation of DAMPs, which may also stimulate the production of IL-1β. There are several mechanisms by which IL-1β secretion can be impacted by exposure to Mn and Ni.
Among the metals with low detection, we observed that women with U detection (n=43) had higher concentrations of IL-1β. The immune system is sensitive to U exposure as evidenced by induction of intestinal chemokine production, elevated kidney toxicity, and altered lymphocyte profiles observed in mice after oral exposures [68,69]. Additionally, Cr detection (n=57) in our study sample was associated with increased TNF-α, and W detection (n=71) was associated with decreased IL-10. Mechanistic studies have also shown that Cr and W can elicit immune responses. For example, oral Cr exposure in mice was accompanied with increased mRNA levels of TNF-α, IL-6, and IL-1β in tissues samples from the liver and spleen [70]. Another experimental study of oral tungstate exposure in mice observed immunosuppressive changes in T-cell populations in splenic tissue [71]. Although there was low detection of these metals in our study sample, these findings along with the observations we observed in our study suggest that even low exposure may have immunotoxic consequences during pregnancy and future studies should continue to conduct exposure assessment of these metals.
We observed a large positive effect estimate between Se and CRP, but no significant association with any of the cytokines. Jamilian and colleagues [36] conducted a randomized clinical trial in Iran (n=40) of Se supplementation and reported no significant differences between Se supplementation during pregnancy and gene expression of multiple cytokines, including IL-1β and TNF-α, which is also similar to what we observed with circulating plasma IL-1β and TNF-α in our study. It is interesting that Se was associated with CRP, while not with any other pro-inflammatory biomarkers. Perhaps Se may elicit non-specific inflammation through pathways aside from cytokine signaling and further investigation of the maternal immune system repertoire is required to test this Se hypothesis.
Cu participates in immune system functions and maintenance, including modulation of Tcell functions and proliferation [72]. Although TNF-α is produced predominantly by macrophages, changes in TNF-α may be a biomarker of shifting profiles of Tcell populations resulting from elevated Cu concentrations. We also observed one anti-inflammatory association between Tl exposure and TNF-α. This relationship is less clear, and is not aligned with our initial hypothesis, because high exposures to Tl has been reported to be associated with several adverse reproductive outcomes [27]. One possible explanation lies within our uncertainty of other immune signaling molecules that Tl might be acting through that could result in this inverse signal with TNF-α. However, both of the associations we observed with Cu and Tl did not meet the q-value cutoff of 0.1, so these relationships may also due to chance and should be tested in another independent sample. Measurement of other immunological signaling molecules may help explain the nuances of these findings for Cu and Tl.
The limitations of this study help contextualize our findings and highlight opportunities for future studies. First, we only measured urinary trace metals from one single time point during pregnancy, which limits our capacity to interrogate long-term exposures. Many of the metals we measured have a half-life that exceeds several days; therefore future studies should consider conducting analysis on blood metals for comparison with findings of urinary trace metals measurements. Future studies should also consider conducting repeated measures analyses of urinary trace metals during pregnancy to assess potential windows of vulnerability. Another limitation of our study is that we measured a small fraction of the circulating immune biomarker repertoire; therefore, we were unable to evaluate the effects of trace metals across the entire maternal immune system. Our study design for primary analyses at visit 3 is cross-sectional; therefore, we cannot discount the possibility of reverse causation from our results. For example, inflammation from other factors such as infection or dietary allergens may result in altered gut permeability and renal excretion, which could result in increased urinary concentrations of trace metals. Furthermore, previous studies have reported differences in cytokine profiles across gestation [73,74], therefore it is possible that the cross-sectional design of this study may not be capturing gestational age-specific effects.
This study contained several strengths. In a well-characterized longitudinal pregnancy cohort, we measured an extensive panel of trace metals, which provides a robust exposure assessment of potential risk factors. Another strength was our use of high-sensitivity assays of multiple immune markers (pro- and anti-inflammatory) in plasma at two time points in pregnancy to assess immune disruption. Furthermore, we utilized a second time point measurement of immune biomarkers to test the robustness of our main findings. We observed the greatest number of single-pollutant associations with IL-1β. We integrated two unique and complementary statistical methods to analyze multiple trace metals as a mixture in association with IL-1β. Few studies have utilized both shrinkage estimation (elastic net) and machine learning tools (Bayesian kernel machine regression) to compare mixtures analysis results. The robust exposure assessment and statistical methods in this study advances the literature on applications for environmental mixtures analyses.
5. Conclusion
In our study we identified multiple metals that are associated with immune biomarkers of inflammation during pregnancy. Among these associations, Mn clearly held the greatest effect in association with IL-1β. We learned that women with a female fetus largely drove the association between Mn and IL-1β. In conclusion, this study provides evidence that Mn may be an important immunotoxicant at certain concentrations during pregnancy. The effects of Mn exposure should be further investigated, especially in mediation analyses of adverse reproductive and developmental outcomes.
Supplementary Material
Highlights.
Multiple urinary trace metals were associated with immune biomarkers
Manganese, barium, and nickel were positively associated with interleukin-1β
Mixtures analysis identified manganese as most predictive of interleukin-1β
Association between manganese and interleukin-1β was greater in women with a female fetus
Acknowledgements:
Subject recruitment and sample collection was originally funded by Abbott Diagnostics.
Funding:
This work was also supported by National Institute of Environmental Health Sciences, National Institutes of Health (grants R01ES018872, P42ES017198, P30ES017885, P50ES026049, and U2CES026553).
Support for Max Aung was provided in part by a grant from the Robert Wood Johnson Foundation Health Policy Research Scholars program.
Support for Kelly Ferguson was provided by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health.
Abbreviations:
- AIC
Aikaike information criterion
- As
Arsenic
- Ba
Barium
- BMI
Body mass index
- CRP
C-reactive protein
- Cu
Copper
- ERS
Environmental risk score
- GM
Geometric mean
- GSD
Geometric standard deviation
- Hg
Total mercury
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- LOD
limit of detection
- Mn
Manganese
- Mo
Molybdenum
- NHANES
National Health and Nutrition Examination Survey
- Ni
Nickel
- Pb
Lead
- Se
Selenium
- Sn
Tin
- Tl
Thallium
- TNF-α
Tumor necrosis factor- α
- Zn
Zinc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM, Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy, Front. Immunol 5 (2014) 58–7. doi: 10.3389/fimmu.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chau A, Markley JC, Juang J, Tsen LC, Cytokines in the perinatal period - Part I, International Journal of Obstetric Anesthesia. 26 (2016) 39–47. doi: 10.1016/j.ijoa.2015.12.005. [DOI] [PubMed] [Google Scholar]
- [3].Racicot K, Kwon J-Y, Aldo P, Silasi M, Mor G, Understanding the Complexity of the Immune System during Pregnancy, Am J Reprod Immunol. 72 (2014) 107–116. doi: 10.1111/aji.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morelli S, Mandal M, Goldsmith LT, Kashani BN, Ponzio NM, The maternal immune system during pregnancy and its influence on fetal development, Rrb. (2015) 171–20. doi: 10.2147/RRB.S80652. [DOI] [Google Scholar]
- [5].Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM, Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy, Front. Immunol 5 (2014) 58–7. doi: 10.3389/fimmu.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang J, Tan G-J, Han L-N, Bai Y-Y, He M, Liu H-B, Novel biomarkers for cardiovascular risk prediction, J Geriatr Cardiol. 14 (2017) 135–150. doi: 10.11909/j.issn.1671-5411.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Romero R, Gotsch F, Pineles B, Kusanovic JP, Inflammation in Pregnancy: Its Roles in Reproductive Physiology, Obstetrical Complications, and Fetal Injury, Nut. Rev 65 (2007) 194–202. doi: 10.1301/nr.2007.dec.S194-S202. [DOI] [PubMed] [Google Scholar]
- [8].Argüello JM, Raimunda D, González-Guerrero M, Metal Transport across Biomembranes: Emerging Models for a Distinct Chemistry, J. Biol. Chem 287 (2012) 13510–13517. doi: 10.1074/jbc.R111.319343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang D, Zhang J, Jiang W, Cao Z, Zhao F, Cai T, et al. , The role of NLRP3-CASP1 in inflammasome- mediated neuroinflammation and autophagy dysfunction in manganese-induced, hippocampal- dependent impairment of learning and memory ability, Autophagy. 13 (2017) 914–927. doi: 10.1080/15548627.2017.1293766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schmidt M, Goebeler M, Immunology of metal allergies, J Dtsch Dermatol Ges. 13 (2015) 653–660. doi: 10.1111/ddg.12673. [DOI] [PubMed] [Google Scholar]
- [11].Pilones K, Tatum A, Gavalchin J, Gestational exposure to mercury leads to persistent changes in T-cell phenotype and function in adult DBF 1mice, Journal of Immunotoxicology. 6 (2009) 161–170. doi: 10.1080/15476910903084021. [DOI] [PubMed] [Google Scholar]
- [12].Li N, Liu X, Zhang P, Qiao M, Li H, Li X, et al. , The effects of early life lead exposure on the expression of interleukin (IL) 1β, IL-6, and glial fibrillary acidic protein in the hippocampus of mouse pups, Hum Exp Toxicol. 34 (2014) 357–363. doi: 10.1177/0960327114529451. [DOI] [PubMed] [Google Scholar]
- [13].Hamilton RF Jr., Buford M, Xiang C, Wu N, Holian A, NLRP3 inflammasome activation in murine alveolar macrophages and related lung pathology is associated with MWCNT nickel contamination, Inhalation Toxicology. 24 (2012) 995–1008. doi: 10.3109/08958378.2012.745633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hanson ML, Holásková I, Elliott M, Brundage KM, Schafer R, Barnett JB, Prenatal cadmium exposure alters postnatal immune cell development and function, Toxicology and Applied Pharmacology. 261 (2012) 196–203. doi: 10.1016/j.taap.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kasten-Jolly J, Lawrence DA, The cationic (calcium and lead) and enzyme conundrum, Journal of Toxicology and Environmental Health, Part B. 21 (2019) 400–413. doi: 10.1080/10937404.2019.1592728. [DOI] [PubMed] [Google Scholar]
- [16].ATSDR, Arsenic Public Health Statement, (2007) 1–10.
- [17].ATSDR, Barium Public Health Statement, (2007) 1–6.
- [18].ATSDR, Toxicological Profile for Molybdenum, (2017) 1–276. [PubMed]
- [19].ATSDR, Copper Public Health Statement, (2004) 1–7.
- [20].ATSDR, Lead Public Health Statement, (2007) 1–13.
- [21].ATSDR, Manganese Public Health Statement, (2012) 1–10.
- [22].ATSDR, Mercury Public Health Statement, (1999) 1–20.
- [23].ATSDR, Nickel Public Health Statement, (2005) 1–7.
- [24].ATSDR, Selenium Public Health Statement, (2003) 1–8.
- [25].ATSDR, Tin Compounds Public Health Statement, (2005) 1–7.
- [26].ATSDR, Zinc Public Health Statement, (2005) 1–7.
- [27].ATSDR, Thallium Public Health Statement, (1992) 1–4.
- [28].Pachana K, Wattanakornsiri A, Nanuam J, Heavy Metal Transport and Fate in the Environmental Compartments, NU. International Journal of Science. 7 (2010) 1–11. [Google Scholar]
- [29].Rahman A, Kumarathasan P, Gomes J, Infant and mother related outcomes from exposure to metals with endocrine disrupting properties during pregnancy, Sci. Total Environ. 569–570 (2016) 1022–1031. doi: 10.1016/j.scitotenv.2016.06.134. [DOI] [PubMed] [Google Scholar]
- [30].Mistry HD, Kurlak LO, Young SD, Briley AL, Broughton Pipkin F, Baker PN, et al. , Maternal selenium, copper and zinc concentrations in pregnancy associated with small-for gestational-age infants, Maternal & Child Nutrition. 10 (2012) 327–334. doi: 10.1111/j.1740-8709.2012.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rayman MP, Wijnen H, Vader H, Kooistra L, Pop V, Maternal selenium status during early gestation and risk for preterm birth, Canadian Medical Association Journal. 183 (2011) 549–555. doi: 10.1503/cmaj.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tsuzuki S, Morimoto N, Hosokawa S, Matsushita T, Associations of Maternal and Neonatal Serum Trace Element Concentrations with Neonatal Birth Weight, PLoS ONE. 8 (2013) e75627–4. doi: 10.1371/journal.pone.0075627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wai K, Mar O, Kosaka S, Umemura M, Watanabe C, Prenatal Heavy Metal Exposure and Adverse Birth Outcomes in Myanmar: A Birth-Cohort Study, Ijerph. 14 (2017) 1339–13. doi: 10.3390/ijerph14111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cheng L, Zhang Bin, Huo W, Cao Z, Liu W, Liao J, et al. , Fetal exposure to lead during pregnancy and the risk of preterm and early-term deliveries, International Journal of Hygiene and Environmental Health. 220 (2017) 984–989. doi: 10.1016/j.ijheh.2017.05.006. [DOI] [PubMed] [Google Scholar]
- [35].Kim SS, Meeker JD, Carroll R, Zhao S, Mourgas MJ, Richards MJ, et al. , Urinary trace metals individually and in mixtures in association with preterm birth, Environment International. 121 (2018) 582–590. doi: 10.1016/j.envint.2018.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bailey KA, Laine J, Rager JE, Sebastian E, Olshan A, Smeester L, et al. , Prenatal Arsenic Exposure and Shifts in the Newborn Proteome: Interindividual Differences in Tumor Necrosis Factor (TNF)-Responsive Signaling, Toxicological Sciences. 139 (2014) 328–337. doi: 10.1093/toxsci/kfu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jamilian M, Samimi M, Afshar Ebrahimi F, Aghadavod E, Mohammadbeigi R, Rahimi M, et al. , Effects of Selenium Supplementation on Gene Expression Levels of Inflammatory Cytokines and Vascular Endothelial Growth Factor in Patients with Gestational Diabetes, Biol Trace Elem Res. 181 (2018) 199–206. doi: 10.1007/s12011-017-1045-8. [DOI] [PubMed] [Google Scholar]
- [38].Zhang J-P, Li F, Yu X-W, Sheng Q, Shi X-W, Zhang X-W, Trace elements and cytokine profile in cytomegalovirus-infected pregnancies: a controlled study, Gynecol Obstet Invest. 65 (2008) 128–132. doi: 10.1159/000110013. [DOI] [PubMed] [Google Scholar]
- [39].Nyland JF, Wang SB, Shirley DL, Santos EO, Ventura AM, de Souza JM, et al. , Fetal and maternal immune responses to methylmercury exposure A cross-sectional study, Environmental Research. 111 (2011) 584–589. doi: 10.1016/j.envres.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kelley AS, Banker M, Goodrich JM, Dolinoy DC, Burant C, Domino SE, et al. , Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation, Scientific Reports. 9 (2019) 5422. doi: 10.1038/s41598-019-41134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nyland JF, Fillion M, Barbosa F Jr., Shirley DL, Chine C, Lemire M, et al. , Biomarkers of Methylmercury Exposure Immunotoxicity among Fish Consumers in Amazonian Brazil, Environ Health Perspect. 119 (2011) 1733–1738. doi: 10.1289/ehp.1103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McElrath TF, Lim KH, Pare E, Rich-Edwards J, Pucci D, Troisi R, et al. , Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy, The American Journal of Obstetrics & Gynecology. 207 (2012) 407.e1–407.e7. doi: 10.1016/j.ajog.2012.08.010. [DOI] [PubMed] [Google Scholar]
- [43].Ferguson KK, McElrath TF, Ko Y-A, Mukherjee B, Meeker JD, Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth, Environment International. 70 (2014) 118–124. doi: 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, et al. , Urinary Phthalate Metabolites in Relation to Preterm Birth in Mexico City, Environ Health Perspect. 117 (2009) 1587–1592. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wei S-Q, Fraser W, Luo Z-C, Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review, Obstet Gynecol. 116 (2010) 393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- [46].Taylor BD, Ness RB, Klebanoff MA, Zoh R, Bass D, Hougaard DM, et al. , First and second trimester immune biomarkers in preeclamptic and normotensive women, Pregnancy Hypertension: an International Journal of Women’s Cardiovascular Health. 6 (2016) 388–393. doi: 10.1016/j.preghy.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Boyle AK, Rinaldi SF, Norman JE, Stock SJ, Preterm birth: Inflammation, fetal injury and treatment strategies, Journal of Reproductive Immunology. 119 (2017) 62–66. doi: 10.1016/j.jri.2016.11.008. [DOI] [PubMed] [Google Scholar]
- [48].Kumar A, Begum N, Prasad S, Agarwal S, Sharma S, IL-10, TNF-α & IFN-γ: potential early biomarkers for preeclampsia, Cell. Immunol 283 (2013) 70–74. doi: 10.1016/j.cellimm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- [49].Ferguson KK, McElrath TF, Chen Y-H, Mukherjee B, Meeker JD, Longitudinal Profiling of Inflammatory Cytokines and C-reactive Protein during Uncomplicated and Preterm Pregnancy, Am J Reprod Immunol. 72 (2014) 326–336. doi: 10.1111/aji.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Aung MT, Ferguson KK, Cantonwine DE, Bakulski KM, Mukherjee B, Loch-Caruso R, et al. , Associations between maternal plasma measurements of inflammatory markers and urinary levels of phenols and parabens during pregnancy: A repeated measures study, Science of the Total Environment, the. 650 (2019) 1–10. doi: 10.1016/j.scitotenv.2018.08.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Richardson DB, Rzehak P, Klenk J, Weiland SK, Analyses of Case–Control Data for Additional Outcomes, Epidemiology. 18 (2007) 441–445. doi: 10.1097/EDE.0b013e318060d25c. [DOI] [PubMed] [Google Scholar]
- [52].Benjamini Y, Hochberg Y, Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing, Journal of the Royal Statistical Society: Series B (Methodological). 57 (1995) 289–300. [Google Scholar]
- [53].Zou H, Zhang HH, On the adaptive elastic-net with a diverging number of parameters, Ann. Statist 37 (2009) 1733–1751. doi: 10.1214/08-AOS625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Park SK, Zhao Z, Mukherjee B, Construction of environmental risk score beyond standard linear models using machine learning methods: application to metal mixtures, oxidative stress and cardiovascular disease in NHANES, (2017) 1–17. doi: 10.1186/s12940-017-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. , Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures, Biostatistics. 16 (2014) 493–508. doi: 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Arbuckle TE, Liang CL, Morisset A-S, Fisher M, Weiler H, Cirtiu CM, et al. , Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study, Chemosphere. 163 (2016) 270–282. doi: 10.1016/j.chemosphere.2016.08.023. [DOI] [PubMed] [Google Scholar]
- [57].Xia W, Zhou Y, Zheng T, Zhang Bin, Bassig BA, Li Y, et al. , Maternal urinary manganese and risk of low birth weight: a case–control study, BMC Public Health. (2016) 1–9. doi: 10.1186/s12889-016-2816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chiu Y-HM, Henn BC, Hsu H-HL, Pendo MP, Coull BA, Austin C, et al. , Sex differences in sensitivity to prenatal and early childhood manganese exposure on neuromotor function in adolescents, Environmental Research. 159 (2017) 458–465. doi: 10.1016/j.envres.2017.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kresovich JK, Bulka CM, Joyce BT, Vokonas PS, Schwartz J, Baccarelli AA, et al. , The Inflammatory Potential of Dietary Manganese in a Cohort of Elderly Men, Biol Trace Elem Res. 183 (2017) 49–57. doi: 10.1007/s12011-017-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Enninga EAL, Nevala WK, Creedon DJ, Markovic SN, Holtan SG, Fetal Sex-Based Differences in Maternal Hormones, Angiogenic Factors, and Immune Mediators During Pregnancy and the Postpartum Period, Am J Reprod Immunol. 73 (2014) 251–262. doi: 10.1111/aji.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Finley JW, Johnson PE, Johnson LK, Sex affects manganese absorption and retention by humans from a diet adequate in manganese, Am. J. Clin. Nutr 60 (1994) 949–955. doi: 10.1093/ajcn/60.6.949. [DOI] [PubMed] [Google Scholar]
- [62].Lorente-Pozo S, Parra-Llorca A, Torres B, Torres-Cuevas I, Nuñez-Ramiro A, Cernada M, et al. , Influence of Sex on Gestational Complications, Fetal-to-Neonatal Transition, and Postnatal Adaptation, Front. Pediatr 6 (2018) 1050–8. doi: 10.3389/fped.2018.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lopez-Castejon G, Brough D, Understanding the mechanism of IL-1β secretion, Cytokine Growth Factor Rev. 22 (2011) 189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, et al. , A Role for the Inflammasome in Spontaneous Labor at Term, Am J Reprod Immunol. 11 (2016) n/a–n/a. doi: 10.1111/aji.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Martín-Sánchez F, Diamond C, Zeitler M, Gomez AI, Baroja-Mazo A, Bagnall J, et al. , Inflammasome-dependent IL-1β release depends upon membrane permeabilisation, Cell Death Differ. 23 (2016) 1219–1231. doi: 10.1038/cdd.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sarkar S, Malovic E, Harischandra DS, Ngwa HA, Ghosh A, Hogan C, et al. , Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes, NeuroToxicology. 64 (2018) 204–218. doi: 10.1016/j.neuro.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Milnerowicz H, Ściskalska M, Dul M, Pro-inflammatory effects of metals in persons and animals exposed to tobacco smoke, Journal of Trace Elements in Medicine and Biology. 29 (2015) 1–10. doi: 10.1016/j.jtemb.2014.04.008. [DOI] [PubMed] [Google Scholar]
- [68].Hao Y, Ren J, Liu J, Yang Z, Liu C, Li R, et al. , Immunological changes of chronic oral exposure to depleted uranium in mice, Toxicology. 309 (2013) 81–90. doi: 10.1016/j.tox.2013.04.013. [DOI] [PubMed] [Google Scholar]
- [69].Dublineau I, Grison S, Linard C, Baudelin C, Dudoignon N, Souidi M, et al. , Short-term Effects of Depleted Uranium on Immune Status in Rat Intestine, Journal of Toxicology and Environmental Health, Part A. 69 (2006) 1613–1628. doi: 10.1080/15287390600629825. [DOI] [PubMed] [Google Scholar]
- [70].Jin Y, Liu L, Zhang S, Tao B, Tao R, He X, et al. , Chromium alters lipopolysaccharide-induced inflammatory responses both in vivo and in vitro, Chemosphere. 148 (2016) 436–443. doi: 10.1016/j.chemosphere.2016.01.057. [DOI] [PubMed] [Google Scholar]
- [71].Osterburg AR, Robinson CT, Mokashi V, Stockelman M, Schwemberger SJ, Chapman G, et al. , Oral tungstate (Na2WO4) exposure reduces adaptive immune responses in mice after challenge, Journal of Immunotoxicology. 11 (2014) 148–159. doi: 10.3109/1547691X.2013.816394. [DOI] [PubMed] [Google Scholar]
- [72].Wintergerst ES, Maggini S, Hornig DH, Contribution of Selected Vitamins and Trace Elements to Immune Function, Ann Nutr Metab. 51 (2007) 301–323. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- [73].Denney JM, Nelson EL, Wadhwa PD, Waters TP, Mathew L, Chung EK, et al. , Longitudinal modulation of immune system cytokine profile during pregnancy, Cytokine. 53 (2011) 170–177. doi: 10.1016/j.cyto.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Curry AE, Vogel I, Skogstrand K, Drews C, Schendel DE, Flanders WD, et al. , Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors, Journal of Reproductive Immunology. 77 (2008) 152–160. doi: 10.1016/j.jri.2007.06.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


