Abstract
Background
Frequent occurrence of paravalvular leak (PVL) after transcatheter aortic valve replacement (TAVR) was the main concern with early-generation devices and focused technological improvements. Current systematic review and meta-analysis sought to compare outcomes of TAVR for severe native valve stenosis with next-generation devices: Lotus and Sapien 3.
Methods
Electronic databases were screened for studies comparing outcomes of TAVR with Lotus and Sapien 3. In a random-effects meta-analysis, the pooled incidence rates of procedural, clinical and functional outcomes according to VARC-2 definitions were assessed.
Results
Eleven observational studies including 2,836 patients (Lotus N=862 vs. Sapien 3 N=1,974) met inclusion criteria. No differences were observed regarding composite endpoints—device success and early safety. Similarly, 30-day mortality, major vascular complications, acute kidney injury and serious bleeding events were similar with both devices. Lotus valve demonstrated 35% reduction of the risk for mild PVL: risk ratio (RR) 0.65, 95% confidence interval (CI): 0.49–0.85, P=0.002; but there were no statistical differences with regard to moderate/severe PVL (RR 0.56, 95% CI: 0.18–1.77, P=0.320). Lotus valves produced significantly higher mean transaortic gradients: mean difference (MD) 0.88 mmHg, 95% CI, 0.24–1.53 mmHg, P=0.007; however, without translation into higher rate of prosthesis-patient mismatch (RR 1.10, 95% CI: 0.82–1.47, P=0.540). As compared to Sapien 3, Lotus device placement was associated with significantly higher rate of permanent pacemaker implantation (RR 2.30, 95% CI: 1.95–2.71, P<0.00001) and cerebrovascular events (RR 1.76, 95% CI: 1.03–2.99, P=0.040).
Conclusions
Lotus valve, as compared with Sapien 3, was associated with lower risk for PVL but higher risk for permanent pacemaker implantation and cerebrovascular events.
Keywords: Meta-analysis, Lotus, Sapien 3, transcatheter aortic valve replacement (TAVR)
Introduction
Since introduction by Cribier in 2002 (1), transcatheter aortic valve replacement (TAVR) has been complementary method to surgical aortic valve replacement (SAVR) in inoperable or high-risk patients with severe symptomatic aortic stenosis. Similar (2) or even lower (3) 1-year mortality rate of TAVR, as compared to SAVR was shown in selected groups of patients. Hence, TAVR is now considered as an alternative treatment option and is recommended not only in inoperable, high or increased risk surgical patients (2-5) but also in intermediate risk individuals (6-9).
Commercially available within initial few years after the first procedure early generation transcatheter valves, despite providing good clinical outcomes, were not free from drawbacks like high rate of conduction abnormalities, vascular complications or more importantly higher incidence of paravalvular leak (PVL), which in turn was associated with increased late mortality and higher rate of adverse clinical incidents as compared to SAVR (10-13).
To minimize these shortcomings technological innovations were developed in next-generations valves, which included among others: balloon-expandable Sapien 3 (Edwards Lifesciences, Irvine, California, USA) and mechanically-expanded, repositionable and retrievable Lotus Valve System (Boston Scientific Corporation, Marlborough, Massachusetts, USA).
The objective of the present investigation was to evaluate and compare short-term results of transcatheter aortic valve implantation with Lotus and Sapien 3 in patients presenting with symptomatic severe native aortic valve stenosis.
Methods
Data sources and search strategy
The systematic review and meta-analysis was performed in accordance to the MOOSE statement (14,15). The MOOSE checklist is available as Table S1. We searched PubMed, ClinicalKey, the Web of Science and Google Scholar as well as congress proceedings from major cardiothoracic and cardiology societies meetings, all until December 2018. Search terms were: “Lotus- or Sapien 3- or Lotus versus Sapien 3-, transcatheter valve”. The literature was limited to articles published in English. References of original articles were reviewed manually and cross-checked. Abstracts were eligible for detailed assessment when were available online and reported outcomes of interest.
Table S1. MOOSE checklist for meta-analyses of observational studies.
| Item No. | Recommendation | Reported on page No. |
|---|---|---|
| Reporting of background should include | ||
| 1 | Problem definition | 2 |
| 2 | Hypothesis statement | 2 |
| 3 | Description of study outcome(s) | 2–3 |
| 4 | Type of exposure or intervention used | 2–3 |
| 5 | Type of study designs used | 2–3 |
| 6 | Study population | 3 |
| Reporting of search strategy should include | ||
| 7 | Qualifications of searchers (e.g., librarians and investigators) | 2 |
| 8 | Search strategy, including time period included in the synthesis and key words | 2, Figure 1 |
| 9 | Effort to include all available studies, including contact with authors | 2 |
| 10 | Databases and registries searched | 2 |
| 11 | Search software used, name and version, including special features used (e.g., explosion) | NA |
| 12 | Use of hand searching (e.g., reference lists of obtained articles) | 2 |
| 13 | List of citations located and those excluded, including justification | Figure 1 |
| 14 | Method of addressing articles published in languages other than English | NA |
| 15 | Method of handling abstracts and unpublished studies | 2 |
| 16 | Description of any contact with authors | NA |
| Reporting of methods should include | ||
| 17 | Description of relevance or appropriateness of studies assembled for assessing the hypothesis to be tested | 2 |
| 18 | Rationale for the selection and coding of data (e.g., sound clinical principles or convenience) | NA |
| 19 | Documentation of how data were classified and coded (e.g., multiple raters, blinding and interrater reliability) | NA |
| 20 | Assessment of confounding (e.g., comparability of cases and controls in studies where appropriate) | 2–3 |
| 21 | Assessment of study quality, including blinding of quality assessors, stratification or regression on possible predictors of study results | 3, Table S2 |
| 22 | Assessment of heterogeneity | 3–7 |
| 23 | Description of statistical methods (e.g., complete description of fixed or random effects models, justification of whether the chosen models account for predictors of study results, dose-response models, or cumulative meta-analysis) in sufficient detail to be replicated | 3 |
| 24 | Provision of appropriate tables and graphics | Yes |
| Reporting of results should include | ||
| 25 | Graphic summarizing individual study estimates and overall estimate | Figures 3-5, Figures S1-S3 |
| 26 | Table giving descriptive information for each study included | Table 1 |
| 27 | Results of sensitivity testing (e.g., subgroup analysis) | NA |
| 28 | Indication of statistical uncertainty of findings | Limitations, 11 |
| Reporting of discussion should include | ||
| 29 | Quantitative assessment of bias (e.g., publication bias) | NA |
| 30 | Justification for exclusion (e.g., exclusion of non-English language citations) | NA |
| 31 | Assessment of quality of included studies | Limitations, 11, Table S2 |
| Reporting of conclusions should include | ||
| 32 | Consideration of alternative explanations for observed results | Discussion, 9–11 |
| 33 | Generalization of the conclusions (i.e., appropriate for the data presented and within the domain of the literature review) | 10–11 |
| 34 | Guidelines for future research | 11 |
| 35 | Disclosure of funding source | No funding |
From Stroup DF, Berlin JA, Morton SC, et al. for the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. Meta-analysis of Observational Studies in Epidemiology. A Proposal for Reporting. JAMA 2000;283:2008-12. NA, not reported.
Selection criteria and quality assessment
Studies were included if having met all of the following criteria: (I) human study; (II) study or study arms comparing directly or indirectly strategy of TAVR with Lotus and Sapien 3. Studies were excluded if: (I) in-vitro study; (II) not reporting outcomes of interest. No restrictions regarding type of the study, number of patients included or characteristic of the population were imposed.
Two reviewers (M Gozdek and J Ratajczak) selected the studies for the inclusion, extracted studies and patients’ characteristics of interest and relevant outcomes. Two authors (M Gozdek and J Ratajczak) independently assessed the trials’ eligibility and risk of bias. Any divergences were resolved by consensus.
Quality of observational studies was appraised with ROBINS-I (Risk of Bias in Nonrandomised Studies-of Interventions), a tool used for assessment of the bias (the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest) in cohort studies included in a systematic review and/or meta-analysis (16).
Endpoints selection
Endpoints were established according to the Valve Academic Research Consortium-2 (VARC-2) definitions (17). Procedural outcomes of interest were: use of more than 1 prosthesis during initial implantation and repeat procedure for valve-related dysfunction in 30 days. Clinical endpoints assessed included: permanent pacemaker implantation (PPI), major vascular complications (MVC), serious bleeding (life-threatening and/or major), acute kidney injury (AKI), cerebrovascular events (CVE) and 30-day mortality. Functional outcomes were: mean transprosthetic gradient, prosthesis-patient mismatch (PPM), mild and moderate to severe PVL. Composite endpoints were: device success and early safety. Additionally, procedure duration, rates of predilatation and postdilatation as well as contrast volume and other non-VARC-2 endpoints, were also considered. To assess PPM incidence, we pooled data expressed by authors as indexed effective orifice area (iEOA) <0.85 cm2/m2 and as a mean transaortic gradient >20 mmHg. We also included data for analysis in cases where the authors only pointed out PPM without specifying values and units.
Statistical analysis
Data were analysed according to intention-to-treat principle wherever applicable. Risk ratios (RRs) and 95% confidence intervals (95% CIs) served as primary index statistics for dichotomous outcomes. For continuous outcomes, mean difference (MD) and corresponding 95% CI were calculated using random effects model. To overcome the low statistical power of Cochran Q test, the statistical inconsistency test I2 = [(Q_df)/Q] ×100%, where Q is the chi-square statistic and df is degrees of freedom, was used to assess heterogeneity (18). It examines the percentage of inter-study variation, with values ranging from 0% to 100%. An I2 value of less than 40% indicates no obvious heterogeneity, values between 40–70% are suggestive of moderate heterogeneity and I2 more than 70% is considered as high heterogeneity.
Because of high degree of heterogeneity anticipated among the present only nonrandomized trials, an inverse variance (DerSimonian-Laird) random-effects model was applied as a more conservative approach for observational data accounting for between- and within-study variability. Whenever a single study reported median values and interquartile ranges instead of mean and standard deviation (SD), the latter were approximated as described by Wan and colleagues (19). In case there were “0 events” reported in both arms, calculations were repeated, as a sensitivity analysis, using risk difference (RD) and respective 95% CI. Review Manager 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark) was used for statistical computations. P values ≤0.05 were considered statistically significant and reported as two-sided, without adjustment for multiple comparisons.
Results
Study selection
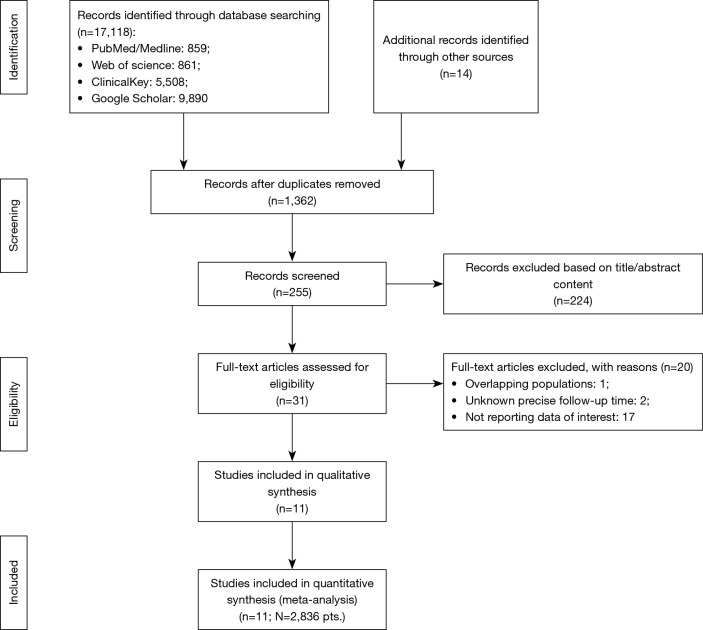
Study selection process and reasons for exclusion of some studies are described in Figure 1. Systematic search of the online databases allowed collection of 255 potentially eligible records that were retrieved for scrutiny. Of those, 244 were further excluded because they were not pertinent to the design of the meta-analysis or did not meet the explicit inclusion criteria. Eleven observational studies (20-30) [among them two multicentre registries (22,25)] enrolling 2,836 patients were eventually included in the analysis. Potential sources of the studies’ bias were analyzed with the use of components recommended by the ROBINS-I tool and the results are enclosed as Table S2. Overall, the studies reported either moderate or serious risk of bias. Most commonly biases arose from participants selection for the study by designated heart teams and subjective distribution of the participants within the study arms by designated operators. Patients were divided into two groups: those treated with Lotus transcatheter valve (N=862) and Sapien 3 transcatheter valve (N=1,974).
Figure 1.
Study selection and inclusion process.
Table S2. ROBINS-I tool bias assessment.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in measurement of interventions | Bias due to departures from intended interventions | Bias due to missing data | Bias in measurement of outcomes* | Bias in selection of reported result | Overall bias |
|---|---|---|---|---|---|---|---|---|
| Abdel-Wahab et al. 2016 (20) | Critical | Serious | Low | Low | Low | Serious | Low | Moderate |
| Fovino et al. 2018 (21) | Critical | Serious | Low | Low | Low | Serious | Low | Moderate |
| Jarr et al. 2017 (23) | Critical | Critical | Low | Moderate | Low | Serious | Low | Serious |
| Marzahn et al. 2018 (24) | Critical | Critical | Low | Low | Low | Moderate | Serious | Serious |
| Pilgrim et al. 2016 (25) | Critical | Critical | Moderate | Low | Low | Moderate | Low | Moderate |
| Schofer et al. 2018 (26) | Critical | Critical | Low | Low | Low | Serious | Low | Moderate |
| Seeger et al. 2017 (27) | Critical | Serious | Moderate | Low | Low | Serious | Low | Moderate |
| Sinning et al. 2017 (28) | Critical | Critical | Low | Low | Low | Serious | Serious | Serious |
| Soliman et al. 2018 (29) | Critical | Critical | Low | Low | Low | Moderate | Serious | Serious |
| van Gils L et al. 2017 (22) | Critical | Critical | Low | Low | Low | Serious | Serious | Serious |
| Wöhrle et al. 2015 (30) | Critical | Critical | Low | Low | Low | Serious | Low | Moderate |
*, when multiple outcomes were reported for a study, the highest level of bias at the outcome level is reported in the table.
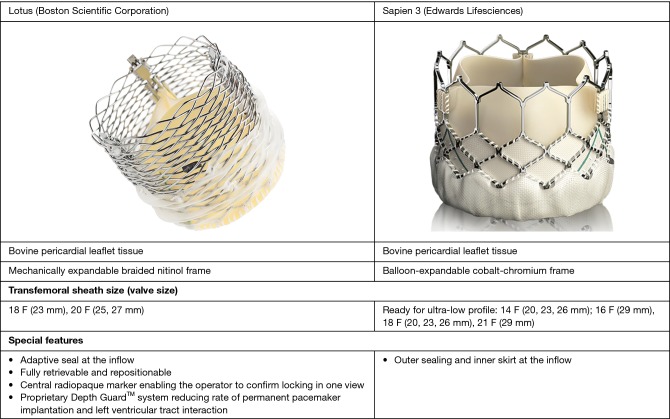
Summary of the valve characteristics is available as Figure 2. Studies’ characteristics as well as definitions or diagnostic criteria for assessed clinical endpoints are reported in Table 1. Table S3 lists selection criteria for the procedure and valve as well as inclusion and exclusion criteria within particular studies. Patients’ baseline characteristics and detailed procedural characteristics are available as Tables S4,S5. All studies reported data on 30-day clinical outcomes and 5 reported data of longer-term follow-up.
Figure 2.
Comparison of valves’ characteristics and special features.
Table 1. Studies’ and patients’ baseline characteristics.
| First author/year | Study period | Design | Intervention | Cohort | Age (years) |
Females (%) |
BMI (kg/m2) |
NYHA III/IV (%) |
STS-PROM (%) | Logistic EuroSCORE (%) | Mean aortic gradient (mmHg) | Aortic annulus diameter (mm) | Access site |
Follow-up (months) | VARC-2 outcomes definitions | ROBINS-I tool bias assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdel-Wahab 2016 (20) | 12.2013–10.2015 | SC, RCS, PM | Sapien 3 | 60 | 81.2±5.1 | 56.7 | 28.3±4.9 | NR | 4.9±3.1 | 17.6±10.8 | 46.1±16.1 | 25.0±2.2 | NR | 12 | Yes | Moderate |
| Lotus | 60 | 80.7±5.2 | 56.7 | 28.2±5.6 | NR | 5.4±3.0 | 17.6±10.5 | 46.1±18.5 | 24.7±2.2 | NR | ||||||
| Fovino 2018 (21) | 08.2013–01.2017 | MCR, PCS, PM | Sapien 3 | 93 | 79.8±5.8 | 41.9& | 26.3±4.3 | 53.8 | 8.7±8.0 | 15.3±10.5& | 42.6±16.9 | 24.8±2.1 | Femoral 100% | 1 | Yes | Moderate |
| Lotus | 93 | 80.5±7.0 | 44.1& | 25.9±4.6 | 51.6 | 9.0±5.2 | 15.9±10.2& | 47.8±14.1 | 24.1±2.3 | Femoral 100% | ||||||
| Jarr 2017 (23) | 08.2014–01.2016 | SC, RCS | CoreValve | 36 | 82.3±4.7 | 73.8 | NR | NR | 6.2±4.7 | NR | 38.7±14.0 | 23.5±1.7& | Femoral 100% | 1 | Yes | Serious |
| Evolut R | 109 | |||||||||||||||
| Sapien 3 | 90 | 81.7±7.3 | 26.7 | NR | NR | 5.3±4.8 | NR | 38.3±14.5 | 25.4±2.3& | Femoral 100% | ||||||
| Lotus | 63 | 81.3±5.1 | 33.3 | NR | NR | 5.6±4.5 | NR | 38.1±13.0 | 24.7±1.9& | Femoral 100% | ||||||
| Marzahn 2018 (24) | 07.2008–05.2015 | SC, RCS | CoreValve | 272 | 80.5±6.1 | 55.7 | 27.8±8.7 | NR | NR | 15.9±10.3 | 42.7±16.6 | NR | Femoral 100% | 12 | Nr | Serious |
| Evolut R | 7 | |||||||||||||||
| Portico | 26 | |||||||||||||||
| DFM | 41 | |||||||||||||||
| Sapien XT | 262 | |||||||||||||||
| Sapien 3 | 234 | |||||||||||||||
| Lotus | 14 | |||||||||||||||
| Pilgrim 2016 (25) | 02.2014–09.2015 | MCR, PCS | Sapien 3 | 815 | 81.9±6.4 | 43.2 | 26.9±5.3 | 66.8 | 5.0±3.8& | 18.9±14.8& | 46.1±21.5 | NR | Femoral 100% | 1 | Yes | Moderate |
| Lotus | 140 | 83.0±5.4 | 46.4 | 26.6±4.8 | 58.6 | 4.1±2.4& | 15.0±8.6& | 49.4±19.5 | NR | Femoral 100% | ||||||
| Schofer 2018 (26) | 2014–2015 | SC, RCS | Sapien 3 | 212 | 80.6±7.2 | 48.1 | 27.3±5.7 | 92.9 | 5.9±5.6 | 16.1±11.0 | 35.0±16.8& | 24.6±2.3 | Femoral 100% | 1 | Yes | Moderate |
| Lotus | 61 | 80.5±7.5 | 57.4 | 28.6±6.4 | 86.9 | 4.8±2.6 | 13.4±8.6 | 40.6±14.2& | 24.0±2.0 | Femoral 100% | ||||||
| Seeger 2017 (27) | 06.2014–2016 | SC, PCS, PM | Sapien 3 | 202 | 80.1±6.4 | 57.4 | 27.1±4.8 | 77.2 | 6.5±5.2 | 14.6±13.0 | 35.0±15.0 | 24.6±2.6 | Femoral 100% | 24 | Yes | Moderate |
| Lotus | 202 | 81.2±5.2 | 56.7 | 26.7±4.8 | 73.1 | 6.8±5.0 | 13.2±12.1 | 36.0±16.0 | 24.3±1.7 | Femoral 100% | ||||||
| Sinning 2017 (28) | 2010–2016 | SC, RCS | Direct Flow Medical | 38 | 80.9±6.3 | 49.2 | NR | NR | 5.6±3.6 | NR | NR | NR | NR | 36 | Yes | Serious |
| CoreValve | 400 | |||||||||||||||
| Evolut R | 114 | |||||||||||||||
| Sapien XT | 48 | |||||||||||||||
| Sapien 3 | 101 | |||||||||||||||
| Lotus | 104 | |||||||||||||||
| Soliman 2018 (29)* | 09.2013–12.2015 | SC, PCS | Sapien 3 | 83 | 80.0±8.0 | 46 | 27.1±5.0 | 74.4 | NR | 15.5±9.5 | NR | 24.9±2.3 | Femoral 90%&; apical 10%& | 1 | Yes | Serious |
| Lotus | 79 | 80.0±7.0 | 56 | 28.0±5.3 | 74.7 | NR | 14.0±9.3 | NR | 24.3±1.7 | Femoral 100%& | ||||||
| van Gils 2017 (22)* | 05.2008–02.2016 | MCR, RCS | CoreValve | 130 | 83.0±6.0 | 39 | 26.0±5.0 | 78& | 7.1±4.4 | NR | NR | NR | Femoral 91%&; apical 1%&; subclavian 8%& | 12 | Yes | Serious |
| Sapien XT | 124 | 83.0±8.0 | 33 | 27.0±4.0 | 83& | 7.0±4.6 | NR | NR | NR | Femoral 79%&; apical 10%&; subclavian 11%& | ||||||
| Sapien 3 | 32 | 81.0±6.0 | 37 | 27.0±4.0 | 53& | 6.0±5.8 | NR | NR | NR | Femoral 78%&; apical 19%&; subclavian 3%& | ||||||
| Lotus | 20 | 83.0±6.0 | 40 | 29.0±7.0 | 74& | 6.3±2.1 | NR | NR | NR | Femoral 100%& | ||||||
| Wöhrle 2015 (30) | 01.2014–06.2014 | SC, RCS | Sapien 3 | 52 | 82.6±6.2& | 48 | NR | 79 | 7.3±5.3 | 17.7±11.8 | 35.0±15.0 | 24.6±1.7 | Femoral 100% | 1 | Yes | Moderate |
| Lotus | 26 | 79.3±5.3& | 62 | NR | 62 | 41.0±17.0 | 25.2±1.7 | Femoral 100% |
*, a possibility of insignificantly overlap of van Gils and Soliman studies (22,29) exists. Fifty-two van Gils’ patients were drawn from four centers and 162 Soliman’s exclusively from 1 shared with van Gils’ center; &, variables that differed significantly. SC, single centre; RCS, retrospective cases series; PM, propensity match; MCR, multicentre registry; PCS, prospective cohort study; BMI, body mass index; NYHA, New York Heart Association; STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; EuroSCORE, European System for Cardiac Operative Risk Evaluation; VARC, Valve Academic Research Consortium; NR, not reported.
Table S3. Studies’ inclusion, exclusion criteria. Choice of procedure and valve type.
| Study (ref) | Inclusion criteria | Exclusion criteria | Selection criteria for the procedure | Selection criteria for the valve |
|---|---|---|---|---|
| Abdel-Wahab et al. 2016 (20) | Patients treated with new generation devices | Patients treated with new generation, self-expanding devices | Not reported | Not reported |
| Fovino et al. 2017 (21) | All consecutive patients with symptomatic severe aortic stenosis undergoing transfemoral TAVI with Sapien 3 in the PUREVALVE registry (n=93) were matched with patients (n=222) undergoing transfemoral TAVI with the Lotus valve included in the RELEVANT study | A life expectancy of less than 1-year, congenital unicuspid or bicuspid aortic valve, severe peripheral artery disease (femoral artery lumen diameter <6.0 mm) and valve-in-valve procedure | Patients were candidate to TAVI by the local Heart Team on the basis of surgical risk score, as well as frailty and presence of comorbidities | Not reported |
| Jarr et al. 2017 (23) | Not reported | Not reported | The decision regarding whether patients were scheduled for TAVI or SAVR was made by institution heart team | The decision regarding the valve type used was at the operator’s discretion |
| Marzahn et al. 2018 (24) | Patients with high-grade aortic stenosis who underwent TAVI |
Not reported | All patients were evaluated for TAVI by an interdisciplinary heart | Prostheses were selected by the implanting cardiologist before intervention based on the patients’ morphology |
| Pilgrim et al. 2016 (25) | Patients with severe aortic stenosis treated with the Edwards Sapien 3 prosthesis or the Lotus valve system | Not reported | Selection of TAVI candidates, device allocation, and periprocedural management was left to the discretion of the operators | Selection of TAVI candidates, device allocation, and periprocedural management was left to the discretion of the operators |
| Schofer et al. 2018 (26) | Consecutive patients with severe symptomatic native aortic valve stenosis who were indicated to receive transfemoral TAVI either by using the Sapien 3 or the Lotus valve | Not reported | Based on an interdisciplinary heart team decision, patients were allocated to TAVI | Not reported |
| Seeger et al. 2017 (27) | Not reported | Not reported | Decision about suitability for TAVI was assessed by the heart team | Sapien 3 was chosen in annulus diameter >27 mm and short distance annulus to coronary ostia; Lotus was chosen in severe left ventricular outflow tract calcification and thrombus in left atrial appendage |
| Sinning et al. 2017 (28) | Not reported | Not reported | Not reported | Not reported |
| Soliman et al. 2018 (29) | Patients who underwent TAVI because of severe aortic stenosis with either the Lotus or the Sapien 3 | Valve-in-valve procedure or TAVI because of aortic regurgitation or patients without echocardiographic follow-up | Eligibility for TAVI and vascular access (i.e., femoral, axillary and apex) was decided during the multidisciplinary valve team discussion | The choice of the valve type was at the operator’s discretion |
| van Gils et al. 2017 (22) | All consecutive patients with preexistent right bundle branch block without a permanent pacemaker | Transcatheter heart valves with <10 cases | Not reported | Not reported |
| Wöhrle et al. 2015 (30) | Patients with severe aortic stenosis in combination with the presence of clinical symptoms | Life expectancy of less than 12 months, congenital unicuspid or bicuspid aortic valve, acute myocardial infarction, stroke or severe gastrointestinal bleeding within the previous 3 months or severe peripheral artery disease favoring the transapical route for TAVI | The decision to perform TAVI was made by a multidisciplinary heart team including an interventional cardiologist and a cardiothoracic surgeon | Not reported |
PUREVALVE, Padua University REVALving Experirnce; RELEVANT, REgistry of Lotus valvE for treatment of aortic VAlve steNosis with Tavr; TAVI, transcatheter aortic valve implantation; SAVR, surgical aortic valve replacement.
Table S4. Patients’ baseline characteristics.
| Study (ref) | Intervention | HT (%) |
DM (%) |
PVD (%) |
CKD (%) |
COPD (%) | PM/ICD (%) | AF (%) |
CAD (%) |
MI history (%) | Stroke history (%) | Heart surgery history (%) | NYHA III/IV | LVEF (%) | Mean aortic gradient (mmHg) | Aortic valve area (cm2) | Aortic annulus diameter (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdel-Wahab et al. 2016 (20) | Sapien 3 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 57.1±8.8 | 46.1±16.1 | 0.7±0.2 | 25.0±2.2 |
| Lotus | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 58.9±9.2 | 46.1±18.5 | 0.7±0.2 | 24.7±2.2 | |
| Fovino et al. 2018 (21) | Sapien 3 | 80.6 | 24.7 | NR | NR | 31.2 | 7.5 | 26.9 | 51.6 | NR | 8.6 | 11.8 | 53.8 | NR | 42.6±16.9 | 0.80±0.21 | 24.8±2.1 |
| Lotus | 82.8 | 28.0 | NR | NR | 29.0 | 11.8 | 22.6 | 46.2 | NR | 12.9 | 16.1 | 51.6 | NR | 47.8±14.1 | 0.71±0.22 | 24.1±23.3 | |
| Jarr et al. 2017 (23) | Sapien 3 | NR | NR | NR | 4.4 | NR | 13.3 | NR | NR | NR | 20 | 11.1 | NR | NR | 38.3±14.5 | 0.75±0.17 | 25.4±2.3 |
| Lotus | NR | NR | NR | 11.1 | NR | 9.5 | NR | NR | NR | 3 | 23.8 | NR | NR | 38.1±13.0 | 0.75±0.35 | 24.7±1.9 | |
| Marzahn et al. 2018 (24) | Sapien 3 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Lotus | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Pilgrimt et al. 2016 (25) | Sapien 3 | 76.8 | 24.5 | 15.5 | NR | 11.2 | 9.8 | NR | 58.5 | 15.0 | 11.2 | 14.0 | 66.8 | 55.1±14.4 | 46.1±21.5 | 0.71±0.23 | NR |
| Lotus | 81.4 | 23.6 | 7.9 | NR | 7.9 | 10.7 | NR | 60.7 | 15.0 | 10.0 | 10.0 | 58.6 | 56.1±12.1 | 49.4±19.5 | 0.66±0.22 | NR | |
| Schofer N et al. 2018 (26) | Sapien 3 | NR | 14.6 | 19.8 | 3.8 | 18.9 | 9.9 | NR | 59.4 | 12.8 | 14.2 | 6.6 | 92.9 | NR | 35.0±16.8 | 0.8±0.2 | 24.6±2.3 |
| Lotus | NR | 16.4 | 19.7 | 8.2 | 13.1 | 11.5 | NR | 61.7 | 14.7 | 21.3 | 3.3 | 86.9 | NR | 40.6±14.2 | 0.8±0.3 | 24.0±2.0 | |
| Seeger et al. 2017 (27) | Sapien 3 | NR | 25.9 | 81.7 | 29.2 | 37.2 | 6.4 | 36.1 | 61.4 | 11.4 | 14.4 | 10.4 | 77.2 | 57.3±15.0 | 35.0±15.0 | 0.78±0.30 | 24.6±2.6 |
| Lotus | NR | 25.9 | 81.7 | 33.3 | 45.1 | 9.4 | 36.6 | 60.7 | 15.9 | 7.9 | 9.5 | 73.1 | 57.0±14.8 | 36.0±16.0 | 0.79±0.33 | 24.3±1.7 | |
| Sinning et al. 2017 (28) | Sapien 3 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Lotus | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Soliman et al. 2018 (29) | Sapien 3 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 74.4 | NR | NR | NR | 24.9±2.3 |
| Lotus | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 74.7 | NR | NR | NR | 24.3±1.7 | |
| van Gils et al. 2017 (22) | Sapien 3 | NR | 41 | 22 | NR | 22 | 0 | 25 | NR | NR | 9 | 37 | 53 | NR | NR | NR | NR |
| Lotus | NR | 45 | 30 | NR | 30 | 0 | 20 | NR | NR | 20 | 25 | 74 | NR | NR | NR | NR | |
| Wöhrle et al. 2015 (30) | Sapien 3 | NR | 31 | 19 | 46 | 67 | 13 | 42 | 40 | 25 | 15 | 13 | 79 | NR | 35.0±15.0 | 0.71±0.17 | 24.6±1.7 |
| Lotus | NR | 23 | 23 | 31 | 62 | 8 | 38 | 27 | 8 | 4 | 8 | 62 | NR | 41.0±17.0 | 0.72±0.21 | 25.2±1.7 |
HT, hypertension; DM, diabetes mellitus; PVD, peripheral vascular disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; PM/ICD, pacemaker/implantable cardioverter-defibrillator; AF, atrial fibrillation; CAD, coronary artery disease; MI, myocardial infarction; LVEF, left ventricle ejection fraction; NR, not reported.
Table S5. Procedural characteristics.
| Study (ref) | Intervention | Anesthesia | Access site | Valve sizes implanted (%) | Pre-dilatation (%) |
Post-dilatation (%) | At least one reposition maneuver (%) | Contrast volume (mL) | Procedure duration (minutes) |
|---|---|---|---|---|---|---|---|---|---|
| Abdel-Wahab et al. 2016 (20) | Sapien 3 | NR | NR | 23 mm 22.0, 26 mm 51.0, 29 mm 27.0 | 30 | 8.3 | NA | NR | 57.7±21.6 |
| Lotus | NR | NR | 23 mm 23.0, 25 mm 37.0, 27 mm 40.0 | 33.3 | 0 | NR | NR | 69.3±19.8 | |
| Fovino et al. 2018 (21) | Sapien 3 | General or conscious sedation | Femoral 100% | 23 mm 38.7, 26 mm 47.3, 29 mm 14.0 | 65.3 | 5.3 | NA | 183.0±72.0 | 81.0±22.0 |
| Lotus | General or conscious sedation | Femoral 100% | 23 mm 48.4, 25 mm 31.2, 27 mm 20.4 | 50.1 | 0 | 26.8 | 241.0±100.0 | 107.0±32.0 | |
| Jarr et al. 2017 (23) | Sapien 3 | NR | Femoral 100% | NR | 57 | 0 | NA | NR | 36.0±10.0 |
| Lotus | NR | Femoral 100% | 23 mm 22.6, 25 mm 27.4, 27 mm 50.0 | 28 | 0 | 28.6 | NR | 53.0±17.0 | |
| Marzahn et al. 2018 (24) | Sapien 3 | General 100.0% | Femoral 100% | NR | NR | NR | NA | NR | NR |
| Lotus | General 100.0% | Femoral 100% | NR | NR | NR | NR | NR | NR | |
| Pilgrim et al. 2016 (25) | Sapien 3 | Conscious sedation 61.5%; general 38.5% | Femoral 100% | 23 mm 26.5, 26 mm 43.1, 29 mm 30.4 | 81.8 | NR | NA | 152.6±93.3 | 70.3±33.5 |
| Lotus | Conscious sedation 75.0%; general 25.0% | Femoral 100% | 23 mm 31.4, 25 mm 36.4, 27 mm 32.1 | 31.4 | NR | NR | 177.1±77.1 | 69.8±26.1 | |
| Schofer et al. 2018 (26) | Sapien 3 | Conscious sedation or general | Femoral 100% | NR | 55.7 | 17.5 | NA | 150.3±73.4 | 94.3±41.7 |
| Lotus | Conscious sedation or general | Femoral 100% | NR | 91.8 | 1.6 | 29.5 | 234.4±102.2 | 117.1±58.9 | |
| Seeger et al. 2017 (27) | Sapien 3 | Local anesthesia under conscious sedation | Femoral 100% | 23 mm 37.6, 26 mm 38.1, 29 mm 24.3 | 93.5 | 0 | NA | 80.0±25.0 | NR |
| Lotus | Local anesthesia under conscious sedation | Femoral 100% | 23 mm 19.8, 25 mm 42.1, 27 mm 38.1 | 87.6 | 0 | 47 | 87.0±33.0 | NR | |
| Sinning et al. 2017 (28) | Sapien 3 | NR | NR | NR | NR | NR | NA | NR | NR |
| Lotus | NR | NR | NR | NR | NR | NR | NR | NR | |
| Soliman et al. 2018 (29) | Sapien 3 | General 100.0% | Femoral 90%, apical 10%, | 23 mm 23.0, 26 mm 47.0, 29 mm 30.0 | 22 | 13 | NA | NR | NR |
| Lotus | General 100.0% | Femoral 100% | 23 mm 33.0, 25 mm 37.0, 27 mm 30.0 | 15 | 0 | NR | NR | NR | |
| van Gils et al. 2017 (22) | Sapien 3 | NR | Femoral 78%, apical 19%, subclavian 3% | NR | NR | NR | NA | NR | NR |
| Lotus | NR | Femoral 100% | NR | NR | NR | NR | NR | NR | |
| Wöhrle et al. 2015 (30) | Sapien 3 | Local anesthesia 100% | Femoral 100% | 23 mm 19.0, 26 mm 54.0, 29 mm 27.0 | NR | 0 | NA | 86.0±25.0 | NR |
| Lotus | Local anesthesia 100% | Femoral 100% | 23 mm 27.0, 25 mm 04.0, 27 mm 69.0 | NR | 0 | 38% | 92.0±36.0 | NR |
NA, not applicable; NR, not reported.
Patients characteristic
Groups treated with Lotus and Sapien 3 did not differ regarding patients’ age (P=0.886) and NYHA III/IV status (P=0.300). Lotus group included significantly more female individuals, 50.8% vs. 45.3%, respectively (P=0.010) and significantly more often suffered from chronic kidney disease (24.7% vs. 17.1%, P=0.005). There was a statistically significant difference in Logistic EuroSCORE (17.4±13.3 vs. 14.5±10.2; P<0.0001) and STS risk profile (5.6±4.5 vs. 6.1±4.0; P=0.017) for Sapien 3 and Lotus, respectively. Aortic valve baseline echo-parameters, i.e., mean trans-aortic gradient and effective orifice area were comparable (P=0.670 and P=0.791 respectively). Despite comparable native annulus diameter (24.36±6.24 vs. 24.78±2.31 for Lotus and Sapien 3 respectively, P=0.087) patients in Lotus group received smaller prostheses; mean size of implanted valve was 25.10 mm in Lotus and 25.99 mm in Sapien 3 patients (P<0.001).
Transfemoral access was mostly employed during TAVR procedure (99% Sapien 3 and 100% Lotus) followed by transapical (0.94%) and transsubclavian (0.06%) in Sapien 3 recipients.
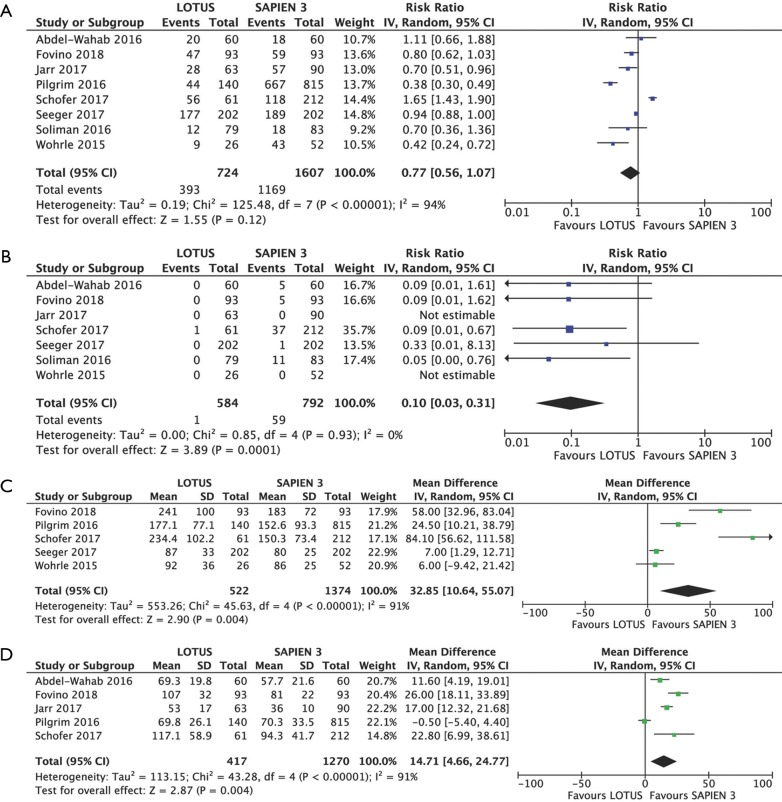
Procedural outcomes
There were no marked differences in rates of predilatation (RR 0.77, 95% CI: 0.56–1.07, P=0.120; I2=94%) while postdilatation was performed less frequently in Lotus recipients (RR 0.10, 95% CI: 0.03–0.31, P=0.0001; I2=0%) (Figure S1A,B). At least one recapture manoeuvre to optimize valve deployment was performed in 37.53% of the Lotus recipients (167 of 445).
The procedures performed with Lotus required significantly greater amount of contrast: 166.3±69.7 vs. 130.4±57.7 mL (MD 32.85, 95% CI: 10.64–55.07, P=0.004) (Figure S1C).
Five studies including 417 Lotus and 1,270 Sapien 3 patients provided data on procedure duration, which was significantly longer in Lotus patients: 83.2±30.8 vs. 67.9±25.8 min (MD 14.71, 95% CI: 4.66–24.77, P=0.004) (Figure S1D).
The need to use more than one prosthesis during initial implantation was very low both in Lotus group (0.17%, 1 of 582 cases) and Sapien 3 group (0.56%, 8 of 1,434 cases) with no statistical difference between the groups (RR 1.02, 95% CI: 0.17–5.96, P=0.990; I2=0%).
Clinical outcomes
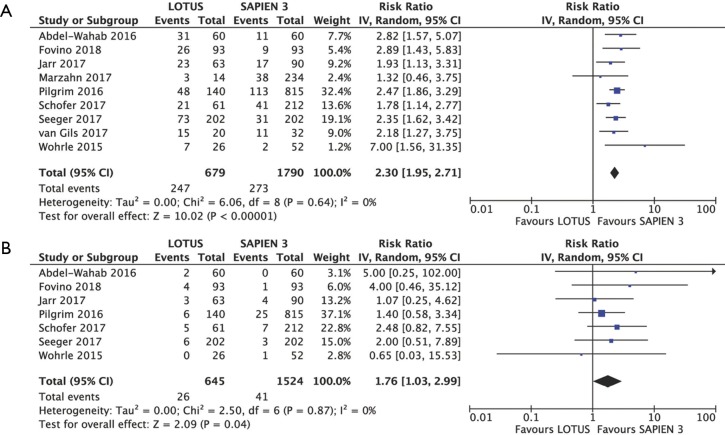
Based on data from nine studies including 2,469 patients (679 Lotus and 1,790 Sapien 3) PPI was required more than twice as often after Lotus as compared to Sapien 3 implantation (RR 2.30, 95% CI: 1.95–2.71, P<0.00001; I2=0%) with corresponding frequency of 36.4% vs. 15.3% respectively (Figure 3A).
Figure 3.
Individual and summary risk ratios with corresponding 95% confidence intervals for the comparison of Lotus vs. Sapien 3 in the analysis of clinical outcomes: permanent pacemaker implantation (A) and cerebrovascular events (B). 95% CI, 95% confidence interval.
Six studies were included for CVE analysis. Lotus patients were 75% more likely to have CVE postoperatively as compared to Sapien 3 (RR 1.76, 95% CI: 1.03–2.99, P=0.04; I2=0%). Corresponding event rates were 4.03% (26 of 645) and 2.69% (41 of 1,524) respectively (Figure 3B).
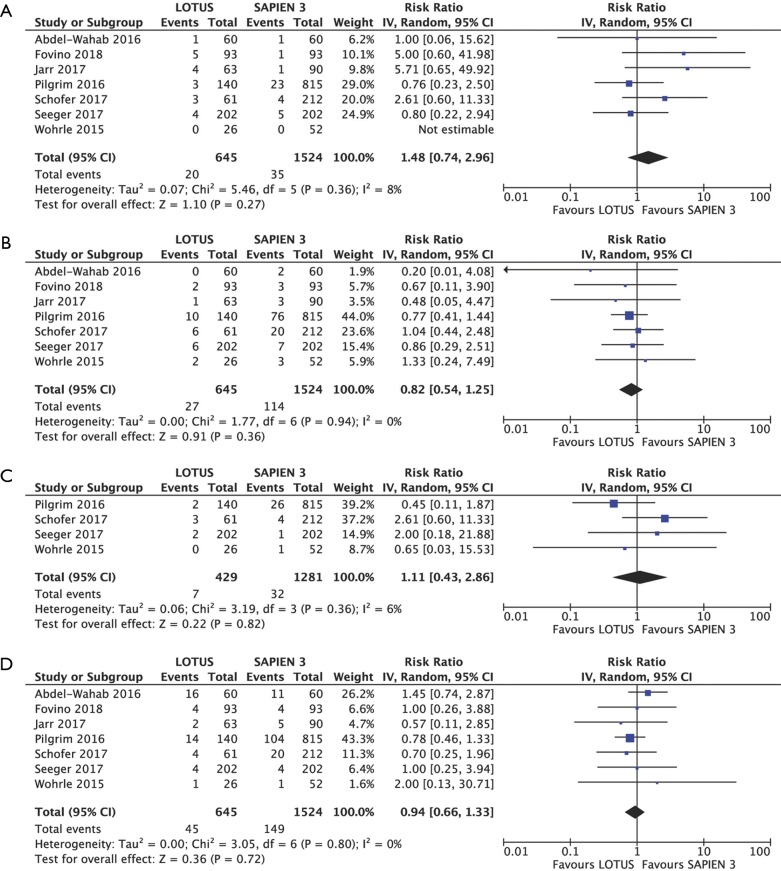
No differences regarding 30-day mortality (RR 1.48, 95% CI: 0.74–2.96, P=0.27; I2=8%), MVC (RR 0.82, 95% CI, 0.54–1.25, P=0.36; I2=0%), AKI (RR 1.11, 95% CI: 0.43–2.86, P=0.82; I2=6%) and occurrence of serious bleeding (RR 0.94, 95% CI: 0.66–1.33, P=0.72; I2=0%) were observed between the two devices. Data from seven studies with a total of 2,169 patients were extracted for analysis of 30-day mortality, MVC, and serious bleeding occurrence. Analysis of AKI included 1,710 individuals from four studies (Figure S2A,B,C,D). Repeat procedures for valve related dysfunction in 30 days were performed only in three Sapien 3 patients (0.82%).
Functional outcomes
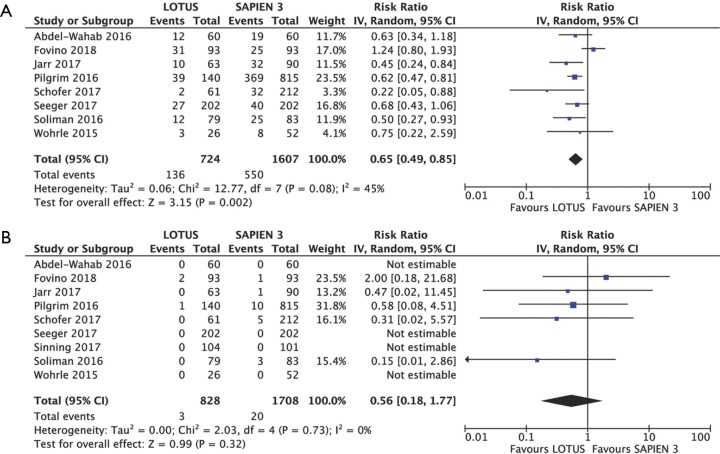
Eight studies including 2,331 patients provided data for PVL analysis. Mild PVL occurred less frequently in Lotus 18.78% (136 of 724) compared to Sapien 3 group 34.23% (550 of 1,607); (RR 0.65, 95% CI: 0.49–0.85, P=0.002; I2=45%) (Figure 4A). Moderate to severe PVL was uncommon in both groups however slightly lower in Lotus as compared to Sapien 3 with corresponding rates of 0.36% (3 of 828) and 1.17% (20 of 1,708) respectively (RR 0.56, 95% CI: 0.18–1.77, P=0.320; I2=0%) (Figure 4B).
Figure 4.
Individual and summary risk ratios with corresponding 95% confidence intervals for the comparison of Lotus vs. Sapien 3 in the analysis of functional outcomes: mild (A) and moderate/severe (B) paravalvular leak. 95% CI, 95% confidence interval.
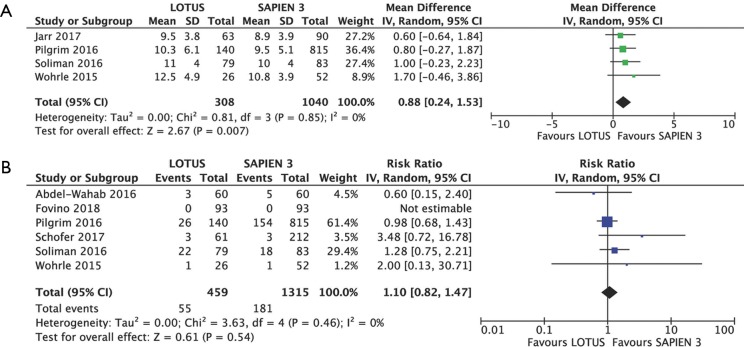
Data regarding postprocedural transaortic gradient came from four studies with 1,040 patients. Mean postprocedural transaortic gradients were higher in Lotus patients (MD 0.88 mmHg, 95% CI: 0.24–1.53 mmHg, P=0.007) but there was no difference in rate of PPM between Lotus and Sapien 3 recipients (RR 1.10, 95% CI: 0.82–1.47, P=0.540; I2=0%). Six studies with 459 Lotus and 1,315 Sapien 3 patients provided data for PPM analysis, which occurred in 11.98% (55 of 459) of patients in Lotus group and 13.76% (181 of 1,315) of patients in Sapien 3 (Figure 5A,B).
Figure 5.
Detailed analysis of individual weighted mean differences (MDs) with corresponding 95% CIs on postoperative mean aortic gradient for the comparison of Lotus vs. Sapien 3 (A) and individual and summary risk ratios with corresponding 95% CIs in the analysis of prosthesis-patient mismatch (B). 95% CI, 95% confidence interval; SD, standard deviation.
Composite endpoints
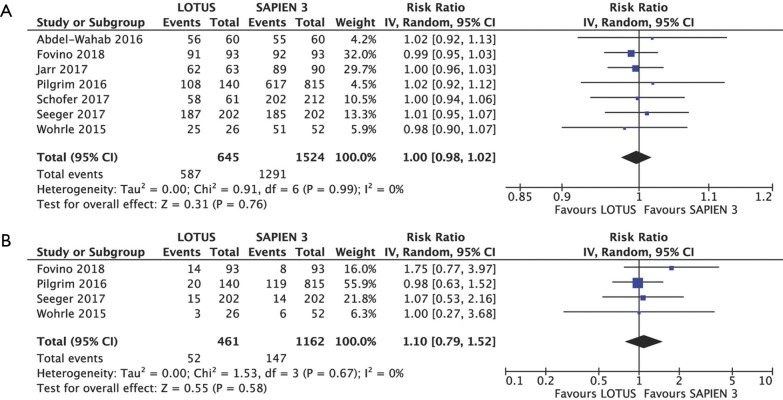
Device success (RR 1.00, 95% CI: 0.98–1.02, P=0.76; I2=0%) and early safety (RR 1.10, 95% CI: 0.79–1.52, P=0.58; I2=0%) were similar for both devices. Overall device success and early safety rate was 86.6% (91.0% Lotus vs. 84.7% Sapien 3) and 12.3% (11.3% Lotus vs. 12.6% Sapien 3), respectively (Figure S3A,B).
Discussion
To the best of our knowledge this is the first systematic review and meta-analysis of observational trials comparing major procedural, short-term clinical and functional outcomes between the Sapien 3 and Lotus, the next-generation valves with external sealing cuffs or skirts with or without mechanisms providing reposition ability to correct a faultily implanted valve which were developed to minimize shortcomings of the early-generation devices. Sapien 3, the next iteration of the balloon-expandable Edwards valve (Edwards Lifesciences, Irvine, California, USA), incorporates an external sealing cuff at the bottom of the stent frame. Having no precursor Lotus Valve System (Boston Scientific Corporation, Marlborough, Massachusetts, USA) is the mechanically-expanded, repositionable and retrievable device contains the adaptive seal at the outer side of frame, located in the left ventricular outflow tract.
Our analysis, by pooling data from eleven studies, demonstrated excellent data regarding short-term performance of both devices. Compared populations of patients seemed to be well balanced with respect to baseline characteristics and severity of underlying valvular disease, although some differences should be highlighted. Patients treated with Lotus were more often female (P=0.010 and received smaller prostheses (P<0.001).
Major finding of the current study is that the Lotus implantation was associated with increased risk of PPI at cost of mild PVL occurrence. We have also demonstrated higher incidence of CVE in Lotus valve recipients. Other clinical endpoints: 30-day mortality, vascular complications, AKI, as well as life threatening and major bleeding, did not differ between the two groups. The use of Lotus valve has also resulted in a higher postprocedural transprosthetic gradient, but it did not translate into a higher frequency of PPM. Procedures with Lotus were significantly longer and required greater amount of contrast volume. Device success and early safety combined endpoints, defined by VARC-2 criteria, were however similar regardless the type of valve implanted.
Current study revealed significantly lower rate of mild PVL with Lotus compared to Sapien 3 (18.78% vs. 34.23% respectively). Moderate to severe aortic regurgitation was seldom in both groups but lower with Lotus (0.36%) than with Sapien 3 (1.17%) yet without statistical significance. PVL of different grades was a frequent complication of early-generation TAVR devices and was associated with worse survival (10,31). Moderate to severe PVL occurred in 7.8% of the self-expandable CoreValve implantation and mild PVL even in one-third of cases (3) in previous studies. The balloon-expandable Sapien XT device was associated with even higher rates of moderate to severe (9.1%) and mild (38.0%) PVL (32). In a randomized study comparing the balloon expandable Edwards Sapien XT with the self-expandable CoreValve device the risk for moderate or severe PVL was greater in the latter group (12.4% vs. 42.5%) (33).
In the next-generations devices, improved by addition an external sealing cuff or a skirt, the frequencies of mild and moderate to severe PVL became significantly lower as compared with the earlier-generation valves. The pooled occurrence of more than mild PVL decreased from 6.9% Sapien XT to 1.6% in Sapien 3 valve as in a meta-analysis by Ando et al. with 2,498 patients (34). Similarly, Swiss TAVI Registry including 598 patients, reported decrease of mild (62.9% to 41.3%) and moderate to severe (5.3% to 1.3%) PVL when Sapien 3 device was implanted (35). The PARTNER II SAPIEN-3 trial, that assessed early outcomes after TAVR in inoperable, high-risk and intermediate-risk patients with severe aortic stenosis, showed moderate to severe PVL in 3.4% and mild in 40.7% of the cases (36). On the other hand, in Lotus TAVR device recipients, rate of all grade PVL was quite low just from the valve’s launch on the market. REPRISE II study reported mild and moderate to severe aortic regurgitation in 13.1% and 2% of Lotus patients (37), whereas the UK LOTUS registry, in 22.8% and 0.8% of patients (38). Our meta-analysis confirms this trend on larger scale with both, mild and moderate to severe PVL rates lower in Lotus as compared to Sapien 3.
Several potential causes of PVL, such as: severe native valve calcification, suboptimal artificial valve sizing, positioning and deployment as well as prosthesis construction itself were universally reported across available literature. Some previous reports noticed that the risk for PVL increases with the extent of landing zone calcification (39-42). Everyday clinical practice shows the Lotus is preferably used in presence of native valve severe calcifications, in particular in case of LVOT involvement. This phenomenon could be explained by higher risk of annular rupture using balloon-expandable valves (43). Schofer et al. demonstrated that the risk for PVL increases with the extent of landing zone calcification also for next-generations devices (26). Thus, PVL was significantly less frequent with the Lotus compared to the Sapien 3 valve with increasing extent of calcification. Additionally, the risk of PVL was reduced by 85% with the Lotus compared to the Sapien 3 valve in these circumstances (26). The fact that Lotus valve implantation has led to significantly lower rate of PVL occurrence, compared to Sapien 3, despite being placed in more demanding environment, could potentially be explained by presence of sealing feature of external cuff. Physical properties of Lotus and Sapien 3 frame seem to be less important since studies showed both valves reaching complete expansion and a circular configuration (44,45).
Long-term follow-up data suggested that even mild PVL was associated with increased late mortality after implantation of the balloon-expandable Edwards Sapien (12) and the CoreValve, early-generation valves (13,46). To date however, no studies exist on late outcomes with Lotus. Therefore, the question if lesser frequency of mild PVL leads to better survival after Lotus implantation remains unanswered.
Another important finding of the present meta-analysis is a significantly higher rate of PPI in Lotus population. In the CHOICE study, that randomized patients to early-generation balloon-expandable Sapien XT and self-expandable CoreValve group, placement of a new permanent pacemaker was necessary in 17.3% and 37.6% respectively (33). Fadahunsi et al. evaluated early-generation transcatheter devices and reported PPI in 25.1% of cases for self-expanding and 4.3% for balloon-expanding devices (47). In study by Adams et al. the self-expandable CoreValve was also related with higher occurrence of PPI (19.8%) (3). Smith et al. reported that PPI was required in 3.8% of cases after the procedure performed with the balloon-expandable Sapien (2).
Changes in the design of next generation devices were supposed to reduce the need for PPI. However, Swiss TAVI Registry revealed the number of PPI increased from 11.0% in Sapien XT to 17.0% in Sapien 3 (35). Similarly, Ando et al. in their meta-analysis comparing early- and next-generation Edwards’ valves, observed higher rate of PPI in Sapien 3 compared with its predecessor (13% vs. 10.5%, P=0.07) (34). Early studies showed high rates of PPI in recipients of Lotus TAVR device. REPRISE II study reported PPI in 28.6% of Lotus patients (37) while the UK LOTUS registry in 31.8% (38). Some factors can drive high frequency of PPI in Lotus recipients, e.g., physical properties of the valve or/and experience, and therefore technical aspects of prosthesis implantation. Learning curve and number of performed procedures seems to play an important role in minimizing the risk of conduction system damage based on the study by Abdel-Wahab et al. who showed trend towards reduced PPI rate in the Lotus group with increased experience (20). Lotus design and thus high radial forces pressing valve’s frame to native annulus and LVOT can affect the conduction system and lead to conduction disturbances. Several previous studies (48-50) indicated the correlation between atrioventricular conduction block and depth of implantation, in particular with depth of more than 6 mm below the native aortic valve annulus, and highlighted the need of high placement of the early-generation self-expanding prosthesis. Similar risk factors were found by Husser et al. (51) in case of Sapien 3. Study by Krackhardt et al. (52) showed that Lotus implantation in a high annulus position was safe and effective—only 10% of patient required PPI in a 30-day follow-up. These results confirmed that implantation technique might be particularly meaningful for the reduction of complications related with the Lotus treatment.
Unlike PVL, landing zone calcification burden showed no significant impact on PPI rate (26).
Previous reports, concerning early-generation valves, suggested the transient nature of TAVR-induced conduction disorders, since up to 50% of the patients with permanent pacemaker were no longer pacemaker dependent at the follow-up (53-55). Alasti et al. reported, only 38% PPI recipients were pacemaker dependent at 1-year follow-up after Lotus implantation (56). The true impact of PPI on long-term outcomes after TAVR remains inconclusive (57,58), however, PPI after TAVR was reported as an independent predictor of 1-year mortality (58) and was also associated with a longer duration of hospitalization and higher rates of rehospitalization at 1 year (53). On the contrary, in the recent analysis including more than 1,500 TAVR procedures the need for PPI did not increase the overall mortality, cardiovascular death or rehospitalization for heart failure within 2 years (59). Moreover, Engborg et al. reported even higher survival in TAVR-patients with permanent pacemaker implanted (60). Furthermore, valve-in-valve TAVR performed with first generation balloon- and self-expandable prosthesis was associated with extremely low rate of PPI (61). Because of artificial ring presence, risk of conduction disturbances is extremely low even for high radial forces bioprostheses.
We have observed higher frequency of CVE in Lotus (4.0%) compared to Sapien 3 (2.70%). Similar results were reported in previous studies regarding both devices. CVE occurred in 3.2% of cases in the UK Lotus registry (38) and in 4.9% in the REPRISE II (37). The PARTNER II SAPIEN-3 trial (36) showed 2.77%, whereas Swiss TAVI Registry revealed 1.2% CVE in Sapien 3 group (35).
Systematic review by Auffret et al. including 64 studies with 72,318 patients showed following CVE predictors: female sex, chronic kidney disease, center experience, new-onset atrial fibrillation (AF) and postdilatation (%) (62). In studies included in the current meta-analysis Lotus recipients were usually initial groups treated by using that device. We have had no data on new-onset AF post TAVR in assessed articles, but there were more females and patients with chronic kidney disease in Lotus groups.
In the current study Sapien 3 recipients more commonly required postdilatation and rapid pacing, which could potentially lead to a hemodynamic unstable situation during the implantation process. Despite this CVE rate was lower in this cohort. Repositioning maneuvers with retrievable valves could potentially contribute to increased CVE occurrence. Our meta-analysis revealed at least one recapture maneuver, to optimize valve deployment, was performed in 37.53% of the Lotus patients (167 of 445).
In study by Schofer et al. (26) more than half of the procedures with the Lotus valve were performed with concomitant use of a cerebral protection device and the risk for CVE did not differ between both groups. On the other hand, dual-filter-based cerebral emboli protection systems could significantly decrease periprocedural ischemic events (63,64). The improvement of implantation technique, along with growing experience, and hence diminish frequency of reposition maneuvers and use of a cerebral protection device might be important aspect of treating patients with Lotus device. Despite statistical significance in the analysis of CVE, differences in risk profiles could have attributed to this result and thus this has to be confirmed in an adequately powered study. In addition, present meta-analysis summarized observational studies alone. The absence of any randomized data inevitably adds to the risk of bias. On the other hand, data are taken from a “real world” which reflects current clinical practice in contrast to randomized trials with highly selected populations. Only half of included studies reported follow-up longer than 1 month. Paucity of data regarding long-term clinical and functional outcomes significantly impedes interpretation of Lotus and Sapien 3 clinical suitability.
Conclusions
Data shows good short-term implantation outcomes of both Lotus and Sapien 3 valves, with no differences in combined endpoints of device success and early safety. Implantation of Lotus was associated with lower risk of mild PVL at cost of higher risk of PPI and CVE. Remaining clinical and functional outcomes were comparable between both valves. Studies of longer follow-ups are sought to evaluate long-term complications and determine their impact on overall survival.
Figure S1.
Procedural outcomes. Individual and summary risk ratios with corresponding 95% confidence intervals (CIs) for the comparison of Lotus vs. Sapien 3 in the analysis of procedural outcomes: predilatation (A) and postdilatation (B). Detailed analysis of individual weighted mean differences (MDs) with corresponding 95% CIs on contrast volume used (C) and procedure duration (D) for the comparison of Lotus vs. Sapien 3. SD, standard deviation.
Figure S2.
Clinical outcomes. Individual and summary risk ratios with corresponding 95% confidence intervals (CIs) for the comparison of Lotus vs. Sapien 3 in the analysis of clinical outcomes: 30-day all-cause mortality (A); major vascular complications (B); acute kidney injury (C) and serious bleeding (D).
Figure S3.
Composite endpoints. Individual and summary risk ratios with corresponding 95% confidence intervals (CIs) for the comparison of Lotus vs. Sapien 3 in the analysis of composite endpoints: device success (A) and early safety (B) according to VARC criteria.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: MK serves as the unpaid editorial board member of Journal of Thoracic Disease from Sep 2018 to Aug 2020. The other authors have no conflicts of interest to declare.
References
- 1.Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002;106:3006-8. 10.1161/01.CIR.0000047200.36165.B8 [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 3.Adams DH, Popma JJ, Reardon MJ, et al. , Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. 10.1056/NEJMoa1400590 [DOI] [PubMed] [Google Scholar]
- 4.Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007;116:755-63. 10.1161/CIRCULATIONAHA.107.698258 [DOI] [PubMed] [Google Scholar]
- 5.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 6.Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387:2218-25. 10.1016/S0140-6736(16)30073-3 [DOI] [PubMed] [Google Scholar]
- 7.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609-20. 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 8.Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321-31. 10.1056/NEJMoa1700456 [DOI] [PubMed] [Google Scholar]
- 9.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2017;70:252-89. 10.1016/j.jacc.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 10.Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol 2013;61:1585-95. 10.1016/j.jacc.2013.01.047 [DOI] [PubMed] [Google Scholar]
- 11.Van Belle E, Juthier F, Susen S, et al. Postprocedural aortic regurgitation in balloon-expandable and self-expandable transcatheter aortic valve replacement procedures: analysis of predictors and impact on long-term mortality: insights from the FRANCE2 Registry. Circulation 2014;129:1415-27. 10.1161/CIRCULATIONAHA.113.002677 [DOI] [PubMed] [Google Scholar]
- 12.Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95. 10.1056/NEJMoa1200384 [DOI] [PubMed] [Google Scholar]
- 13.Jones BM, Tuzcu EM, Krishnaswamy A, et al. Prognostic significance of mild aortic regurgitation in predicting mortality after transcatheter aortic valve replacement. J Thorac Cardiovasc Surg 2016;152:783-90. 10.1016/j.jtcvs.2016.05.023 [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008-12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012;60:1438-54. 10.1016/j.jacc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel-Wahab M, El-Mawardy M, Schwartz B, et al. Comparison of ballon-expandable and mechanically-expanded transcatheter aortic valves: Matched comparison from a single center experience. Paper presented at EuroPCR; May 17-20, 2016; Paris, France. [Google Scholar]
- 21.Fovino LN, Badawy MRA, Fraccaro C, et al. Transfemoral aortic valve implantation with new-generation devices: the repositionable Lotus vs the balloon-expandable Edwards Sapien 3 valve. J Cardiovasc Med (Hagerstown) 2018;19:655-63. 10.2459/JCM.0000000000000705 [DOI] [PubMed] [Google Scholar]
- 22.van Gils L, Tchetche D, Lhermusier T, et al. Transcatheter heart valve selection and permanent pacemaker implantation in patients with pre-existent right bundle branch block. J Am Heart Assoc 2017. doi: . 10.1161/JAHA.116.005028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarr KU, Leuschner F, Meder B, et al. Initial single-center experience with the fully repositionable transfemoral lotus aortic valve system. J Invasive Cardiol 2017;29:30-5. [PubMed] [Google Scholar]
- 24.Marzahn C, Koban C, Seifert M, et al. Conduction recovery and avoidance of permanent pacing after transcatheter aortic valve implantation. J Cardiol 2018;71:101-8. 10.1016/j.jjcc.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 25.Pilgrim T, Stortecky S, Nietlispach F, et al. Repositionable versus balloon-expandable devices for transcatheter aortic valve implantation in patients with aortic stenosis. J Am Heart Assoc 2016;5:e004088. 10.1161/JAHA.116.004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schofer N, Deushl F, Schon G, et al. Comparative analysis of balloon- versus mechanically-expandable transcatheter heart valves considering landing zone calcification. J Cardiol 2018;71:540-6. 10.1016/j.jjcc.2017.09.014 [DOI] [PubMed] [Google Scholar]
- 27.Seeger J, Gonska B, Rottbauer W, et al. Outcome with the repositionable and retrievable Boston Scientific Lotus valve compared with the balloon-expandable Edwards Sapien 3 valve in patients undergoing transfemoral aortic valve replacement. Circ Cardiovasc Interv 2017;10:e004670. 10.1161/CIRCINTERVENTIONS.116.004670 [DOI] [PubMed] [Google Scholar]
- 28.Sinning JM, Stundl A, Mellert F, et al. Comparison of next generation transcatheter heart valves: Angiographic, echocardiographic and hemodynamic evaluation of an extended all-comers study cohort using the dimensionless aortic regurgitation index. J Am Coll Cardiol 2017;69:S585. 10.1016/j.jacc.2017.03.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soliman OI, El Faquir N, Ren B, et al. Comparison of valve performance of the mechanically expanding Lotus and the balloon-expanded Sapien 3 transcatheter heart valves: an observational study with independent core laboratory analysis. Eur Heart J Cardiovasc Imaging 2018;19:157-67. 10.1093/ehjci/jew280 [DOI] [PubMed] [Google Scholar]
- 30.Wöhrle J, Gonska B, Rodewald C, et al. Transfemoral aortic valve implantation with the repositionable Lotus valve compared with the balloon-expandable Edwards Sapien 3 valve. Int J Cardiol 2015;195:171-5. 10.1016/j.ijcard.2015.05.139 [DOI] [PubMed] [Google Scholar]
- 31.Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 2011;123:299-308. 10.1161/CIRCULATIONAHA.110.946533 [DOI] [PubMed] [Google Scholar]
- 32.Kodali S, Pibarot P, Douglas PS, et al. , Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J 2015;36:449-56. 10.1093/eurheartj/ehu384 [DOI] [PubMed] [Google Scholar]
- 33.Abdel-Wahab M, Mehilli J, Frerker C, et al. Comparison of balloon-expandable vs self- expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 2014;311:1503-14. 10.1001/jama.2014.3316 [DOI] [PubMed] [Google Scholar]
- 34.Ando T, Briasoulis A, Holmes AA, et al. Sapien 3 versus Sapien XT prosthetic valves in transcatheter aortic valve implantation: a meta-analysis. Int J Cardiol 2016;220:472-8. 10.1016/j.ijcard.2016.06.159 [DOI] [PubMed] [Google Scholar]
- 35.Binder RK, Stortecky S, Heg D, et al. Procedural results and clinical outcomes of transcatheter aortic valve implantation in Switzerland: an observational cohort study of Sapien 3 versus Sapien XT transcatheter heart valves. Circ Cardiovasc Interv 2015. doi: . 10.1161/CIRCINTERVENTIONS.115.002653 [DOI] [PubMed] [Google Scholar]
- 36.Kodali S, Thourani VH, White J, et al. Early clinical and echocardiographic outcomes after Sapien 3 transcatheter aortic valve replacement in inoperable, high-risk, and intermediate-risk patients with aortic stenosis. Eur Heart J 2016;37:2252-62. 10.1093/eurheartj/ehw112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meredith Am IT, Walters DL, Dumonteil N, et al. Transcatheter aortic valve replacement for severe symptomatic aortic stenosis using a repositionable valve system: 30-day primary endpoint results from the REPRISE II study. J Am Coll Cardiol 2014;64:1339-48. 10.1016/j.jacc.2014.05.067 [DOI] [PubMed] [Google Scholar]
- 38.Rampat R, Khawaja MZ, Byrne J, et al. Transcatheter aortic valve replacement using the repositionable LOTUS valve. JACC Cardiovasc Interv 2016;9:367-72. 10.1016/j.jcin.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 39.Seiffert M, Fujita B, Avanesov M, et al. Device landing zone calcification and its impact on residual regurgitation after transcatheter aortic valve implantation with different devices. Eur Heart J Cardiovasc Imaging 2016;17:576-84. 10.1093/ehjci/jev174 [DOI] [PubMed] [Google Scholar]
- 40.Khalique OK, Hahn RT, Gada H, et al. Quantity and location of aortic valve complex calcification predicts severity and location of paravalvular regurgitation and frequency of post-dilation after balloon-expandable transcatheter aortic valve replacement. JACC Cardiovasc Interv 2014;7:885-94. 10.1016/j.jcin.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 41.Leber AW, Kasel M, Ischinger T, et al. Aortic valve calcium score as a predictor for outcome after TAVI using the CoreValve revalving system. Int J Cardiol 2013;166:652-7. 10.1016/j.ijcard.2011.11.091 [DOI] [PubMed] [Google Scholar]
- 42.Buellesfeld L, Stortecky S, Heg D, et al. Extent and distribution of calcification of both the aortic annulus and the left ventricular outflow tract predict aortic regurgitation after transcatheter aortic valve replacement. EuroIntervention 2014;10:732-8. 10.4244/EIJV10I6A126 [DOI] [PubMed] [Google Scholar]
- 43.Barbanti M, Yang TH, Rodes Cabau J, et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013;128:244-53. 10.1161/CIRCULATIONAHA.113.002947 [DOI] [PubMed] [Google Scholar]
- 44.Rodríguez-Olivares R, Faquir NE, Rahhab Z, et al. Determinants of image quality of rotational angiography for on-line assessment of frame geometry after transcatheter aortic valve implantation. Int J Cardiovasc Imaging 2016;32:1021-9. 10.1007/s10554-016-0889-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodríguez-Olivares R, Rahhab Z, Faquir NE, et al. Differences in frame geometry between balloon-expandable and self-expanding transcatheter heart valves and association with aortic regurgitation. Rev Esp Cardiol 2016;69:392-400. [DOI] [PubMed] [Google Scholar]
- 46.Little SH, Oh JK, Gillam L, et al. Self-Expanding Transcatheter Aortic Valve Replacement Versus Surgical Valve Replacement in Patients at High Risk for Surgery: A Study of Echocardiographic Change and Risk Prediction. Circ Cardiovasc Interv 2016. doi: . 10.1161/CIRCINTERVENTIONS.115.003426 [DOI] [PubMed] [Google Scholar]
- 47.Fadahunsi OO, Olowoyeye A, Ukaigwe A, et al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv 2016;9:2189-99. 10.1016/j.jcin.2016.07.026 [DOI] [PubMed] [Google Scholar]
- 48.Petronio AS, Sinning JM, Van Mieghem N, et al. Optimal implantation depth and adherence to guidelines on permanent pacing to improve the results of transcatheter aortic valve replacement with the Medtronic CoreValve system: the CoreValve prospective, international, post-market ADVANCE-II study. JACC Cardiovasc Interv 2015;8:837-46. 10.1016/j.jcin.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 49.Aktug Ö, Dohmen G, Brehmer K, et al. Incidence and predictors of left bundle branch block after transcatheter aortic valve implantation. Int J Cardiol 2012;160:26-30. 10.1016/j.ijcard.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 50.Binder RK, Webb JG, Toggweiler S, et al. Impact of post-implant Sapien XT geometry and position on conduction disturbances, hemodynamic performance, and paravalvular regurgitation. JACC Cardiovasc Interv 2013;6:462-8. 10.1016/j.jcin.2012.12.128 [DOI] [PubMed] [Google Scholar]
- 51.Husser O, Pellegrini C, Kessler T, et al. Predictors of permanent pacemaker implantations and new-onset conduction abnormalities with the Sapien 3 balloon-expandable transcatheter heart valve. JACC Cardiovasc Interv 2016;9:244-54. 10.1016/j.jcin.2015.09.036 [DOI] [PubMed] [Google Scholar]
- 52.Krackhardt F, Kherad B, Krisper M, et al. Low permanent pacemaker rates following Lotus device implantation for transcatheter aortic valve replacement due to modified implantation protocol. Cardiol J 2017;24:250-8. 10.5603/CJ.a2017.0024 [DOI] [PubMed] [Google Scholar]
- 53.Nazif TM, Dizon JM, Hahn RT, et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (placement of aortic transcatheter valves) trial and registry. JACC Cardiovasc Interv 2015;8:60-9. 10.1016/j.jcin.2014.07.022 [DOI] [PubMed] [Google Scholar]
- 54.Roten L, Stortecky S, Scarcia F, et al. Atrioventricular conduction after transcatheter aortic valve implantation and surgical aortic valve replacement. J Cardiovasc Electrophysiol 2012;23:1115-22. 10.1111/j.1540-8167.2012.02354.x [DOI] [PubMed] [Google Scholar]
- 55.van der Boon RM, Van Mieghem NM, Theuns DA, et al. Pacemaker dependency after transcatheter aortic valve implantation with the self-expanding Medtronic CoreValve System. Int J Cardiol 2013;168:1269-73. 10.1016/j.ijcard.2012.11.115 [DOI] [PubMed] [Google Scholar]
- 56.Alasti M, Rashid H, Rangasamy K, et al. Long-term pacemaker dependency and impact of pacing on mortality following transcatheter aortic valve replacement with the LOTUS valve. Catheter Cardiovasc Interv 2018;92:777-82. 10.1002/ccd.27463 [DOI] [PubMed] [Google Scholar]
- 57.Buellesfeld L, Stortecky S, Heg D, et al. Impact of permanent pacemaker implantation on clinical outcome among patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol 2012;60:493-501. 10.1016/j.jacc.2012.03.054 [DOI] [PubMed] [Google Scholar]
- 58.Dizon JM, Nazif TM, Hess PL, et al. Chronic pacing and adverse outcomes after transcatheter aortic valve implantation. Heart 2015;101:1665-71. 10.1136/heartjnl-2015-307666 [DOI] [PubMed] [Google Scholar]
- 59.Urena M, Webb JG, Tamburino C, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation 2014;129:1233-43. 10.1161/CIRCULATIONAHA.113.005479 [DOI] [PubMed] [Google Scholar]
- 60.Engborg J, Riechel-Sarup C, Gerke O, et al. Effect of permanent pacemaker on mortality after transcatheter aortic valve replacement. Scand Cardiovasc J 2017;51:40-6. 10.1080/14017431.2016.1236982 [DOI] [PubMed] [Google Scholar]
- 61.Gozdek M, Raffa GM, Suwalski P, et al. Comparative performance of transcatheter aortic valve-in-valve implantation versus conventional surgical redo aortic valve replacement in patients with degenerated aortic valve bioprostheses: systematic review and meta-analysis. Eur J Cardiothorac Surg 2018;53:495-504. 10.1093/ejcts/ezx347 [DOI] [PubMed] [Google Scholar]
- 62.Auffret V, Regueiro A, Del Trigo M, et al. Predictors of early cerebrovascular events in patients with aortic stenosis undergoing transcatheter aortic valve replacement. J Am Coll Cardiol 2016;68:673-84. 10.1016/j.jacc.2016.05.065 [DOI] [PubMed] [Google Scholar]
- 63.Van Mieghem NM, Schipper ME, Ladich E, et al. Histopathology of embolic debris captured during transcatheter aortic valve replacement. Circulation 2013;127:2194-201. 10.1161/CIRCULATIONAHA.112.001091 [DOI] [PubMed] [Google Scholar]
- 64.Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following trans-catheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI Randomized Clinical Trial. JAMA 2016;316:592-601. 10.1001/jama.2016.10302 [DOI] [PubMed] [Google Scholar]