Abstract
Objectives
The application of hyaluronic acid (HA) in dental treatments is relatively new, and modified-HA products can be vastly different from each other. This study aims to provide a basis for bridging specific characteristics of HA with its potential applications in dental treatments, evaluating and comparing different types of HA products and for future research on HA applications in dentistry.
Data sources
Information from the existing literature on HA applications has been cited.
Study selection
Furthermore, this study is specifically oriented to provide oral health care providers with a scientific basis for HA use along with the clinical aspects of HA.
Conclusions
Outcomes from existing and future studies cannot be generalised for HA use in dental applications. Therefore, we have proposed a scheme to bridge HA specific characteristics to its applications in dental treatments and compare different HA products used for the same clinical application to identify the most suitable one.
Clinical significance
Highlighting the use of HA in dental treatments and providing a basis for developing new methods, protocols, and products specifically oriented for dentistry.
Keywords: Materials science, Dentistry, Natural product chemistry, Biotechnology, Biochemistry, Hyaluronan, Hyaluronic acid, Dental treatments, Advances in dentistry, Regenerative dentistry, Glycoproteins, Carbohydrates, Extracellular matrix, Polymers
Materials science, Dentistry, Natural product chemistry, Biotechnology, Biochemistry, Hyaluronan, Hyaluronic acid, Dental treatments, Advances in dentistry, Regenerative dentistry, Glycoproteins, Carbohydrates, Extracellular matrix, Polymers.
1. Introduction
Hyaluronic Acid (HA) is a special type of glycosaminoglycan (polysaccharide, carbohydrate) and an interesting biomolecule. It has been used in many medical applications, including cosmetics, aesthetic treatments, and treatment of interstitial cystitis, urinary incontinence, vesicoureteral reflux, and osteoarthritis. Studying the essential molecules of living organisms (including HA) includes knowledge of their sources in nature and methods of synthesis within an organism or artificial synthesis, the study of their chemical and physical structures, biochemical reactions, cellular pathways and interactions, and their association with the organism's developmental stages, nutrition, anatomy, and physiology. Furthermore, it includes the study of their influence on genetics, health, diseases states, gender, and age, and how they are affected by environmental factors. Finally, the same biological molecule can be compared between different organisms (species). It is worth mentioning that biophysics provides the knowledge of the biomechanics of molecules, tissues, and organs, and how these systems work within an organism.
In this study, we have briefly mentioned the characteristics of HA, and focused on the possible applications of HA as a biomolecule along with proposing a scheme to bridge its specific characteristics to its applications in dental treatments and to compare different HA products to identify the most suitable one for particular procedures.
2. Discussion
2.1. Structure and functions of biomolecules
An important characteristic of biomolecules is that they can exist in multiple conformational forms, mainly due to the different types of bonds between their individual elements. For instance, glycogen, starch, and cellulose are all made of glucose units linked by 1–4 glycosidic bonds. In glycogen and starch, the link is at the alpha position, but in cellulose, it is at the beta position. The bond configuration affects the geometry of the molecule resulting in a banded helix for glycogen and starch, so that it occupies minimal volume in the cell as they are intended for energy storage, whereas cellulose forms an extended shape as it is intended to provide mechanical integrity to the cell wall in plants [1]. Thus, we can say that ‘a specific structure is proof of a specific function, whereas a specific function requires a specific structure’ [2].The extra cellular matrix (ECM) is composed of many biomolecules and proteins, all composing a sophisticated network. ECM composition and organisation are tissue-specific and correspond to specific physiological and biochemical functional properties. Naturally, this specificity tends to be expressed as the specific rate of synthesis and degradation in different tissue types and species. For instance, in humans, the HA half-life in the joints, epidermis, and blood is 1–30 weeks, 1–2 days and 2–5 min, respectively [2, 3]. The difference in HA half-life in ECM of different tissues is due to its different conformational forms owing to association with different biological molecules. The ECM stands for the framework where cells can attach themselves and perform their physiological functions [4].
2.2. Importance of HA in human body
HA (C14H21NO11) n is a homogenous unbranched glycosaminoglycan composed of repeated disaccharides. In the human body, HA biopolymer (native HA) constitutes a major component of the extracellular matrix (ECM), and can be found in many different tissues [2, 5]. Native HA, as a biomolecule, is present in oral tissues, such as the gingiva and the periodontal ligaments (synthesised by fibroblasts and keratinocytes), and in low quantities in cementum and the alveolar bone (synthesised by cementoblasts and osteoblasts). Furthermore, HA is present in the saliva with a concentration in the range of 148–1270 ng per milligram of protein in the unstimulated whole saliva [2, 6]. This variation in HA levels is mainly due to variations in the individual diet, oral hygiene, genetics, oral anatomy, health and disease state, and others.
Different concentrations and molecular weights of HA (tissue dependent) triggers different cell responses. In general, high-molecular-weight HA (>5 Million Da) has an anti-angiogenic and immunosuppressive effects. Medium-size HA (2 × 104–1 Million Da) is associated with certain biological processes such as embryogenesis, wound healing and regeneration. Small-size HA (6 × 103 Da–2 x 104 Da) is associated with different cellular processes (e.g. pro-inflammatory, angiogenesis and gene expression). Small strands of HA can act as inducers of the heat shock proteins and an anti-apoptotic agent [7].
HA is naturally synthesised by the proteins (HA synthases), which are of several types in human: HAS1, HAS2, and HAS3 [6,8]. In the human body, the hyaluronidase enzymes, which are also of several types, are responsible for enzymatic degradation of HA. It is estimated that 85% of the HA is turned over in the lymph nodes, and the rest is removed by the liver and kidneys [9, 10]. Complete HA degradation yields CO2, NH3, acetate, and lactate, which are further metabolised by hepatocytes to CO2, H2O, and urea. Native HA molecule along with its receptors, HA synthases, and HA hyaluronidases, are all tissue-specific and dependent on the developmental phase, gender, age, health/disease state, and environmental factors [9, 11, 12, 13].
2.3. Natural sources of HA
HA can be obtained either by extraction from animal tissues (usually rooster combs) or from certain bacterial strains which naturally produce HA (mainly Streptococcus zooepidemicus) or that have been genetically modified to (e.g. Bacillus, Agrobacterium, Escherichia coli, and Lactococcus) produce HA [14]. The process of HA extraction includes the associated risk of having sources of contamination, such as viruses and animal proteins (HA extracted from animal sources), and toxins along with bacterial proteins (HA extracted from bacteria). Such sources of contamination may trigger an immune response. As per recent reports, marine organisms are a possible resource for HA [7, 15, 16].
2.4. Modification of native HA
Although, due to extraction methods used, the conformation of the extracted HA may be different from that of the native HA in vivo, it is the closest form to native HA and can be used for certain medical applications, where such forms of HA are often degraded quickly by hyaluronidases. For some medical applications, the extracted HA must be chemically modified to serve certain cellular or physiochemical properties. A good example is the chemical modification of HA used in intraarticular injections, where the residence time (product-dependent) extends from weeks up to a year after administration of HA into the articular joint.
Generally, the purpose of HA modification is as follows:
-
•
To enhance its resistance to degradation, and thereby enhancing its residence time and the duration of its effects [17].
-
•
To adjust some of its properties, such as viscosity, elasticity and hydrophilicity, to meet certain physiochemical and mechanical properties [18].
-
•
To have the HA in the final form of a gel or hard textured scaffold with specific pore and particle sizes for specific cellular functions (such as cell adherence or migration) [19].
-
•
To create HA-drug conjugates and micelles for sustained or targeted drug release [20, 21].
-
•
To link HA to natural or synthetic compounds (e.g. proteins, natural polymers, liposomes, synthetic polymers, drugs, and so on.) to achieve certain physiochemical or therapeutic properties [22].
There is a wide array of technical and chemical methods used to modify HA, each resulting in specific geometry having certain conformational form and physiochemical characteristics. Those physiochemical characteristics are affected by many factors, including the source of HA, manufacturing technique, HA molecular weight distribution, modification method, chemical agents used during the manufacturing process, purity of HA, and mixing the HA with other materials (if applied). This cumulatively affects the method of administration, degradation, residence time, and possibly the route of elimination of the product. Accordingly, a biocompatibility profile (including purity, toxicity, impurities, etc.) should be established for a modified HA. A famous method is the cross-linking process, in which the HA chains are joined through chemical covalent bonds. Cross-linking processes lead to the formation of 3-dimensional (3D) HA networks having modified physiochemical properties, particularly resistance to degradation, which results in a prolonged life span in the treated tissue [23].
Review of the available literature for HA use in dental applications revealed controversial and contradictory outcomes and variable data due to the large variety of molecular weights, methods of modification, and HA concentrations reported by previous studies [24]. Therefore, the outcomes from existing literature cannot be generalised for HA use in dental applications. In fact, a recently published systematic review [25] concluded that further well-designed randomised clinical trials will be needed to evaluate HA use in various clinical scenarios. As the exact biological functions of HA are still not well understood, there is a necessity to develop new methods to identify its structure in living tissues, interactions with other molecules and receptors and to better understand its association with cellular functions [2].
From clinical aspect, future studies should seek to compare different HA formulations used for the same clinical application to identify the most suitable one. To our knowledge, only two published studies have tested different HA preparations for dental applications. The first study (Vandana and Singh, 2019) [26] tested the effect of different HA concentrations on interdental papillary deficiency treatment and the second study (Asparuhova et al., 2018) [27] tested two different HA preparations on primary human oral fibroblasts.
Researchers should make all efforts to study, test, and report HA characteristics and modifications to enable other researchers compare different types of HA. In addition to these characteristics, it is important to study and report the microscopical appearance (transmission electron microscopy for its supramolecular organisation and scanning electron microscopy for its surface topography, fibrillar organization, and porosity) and its UV absorption spectra [2].
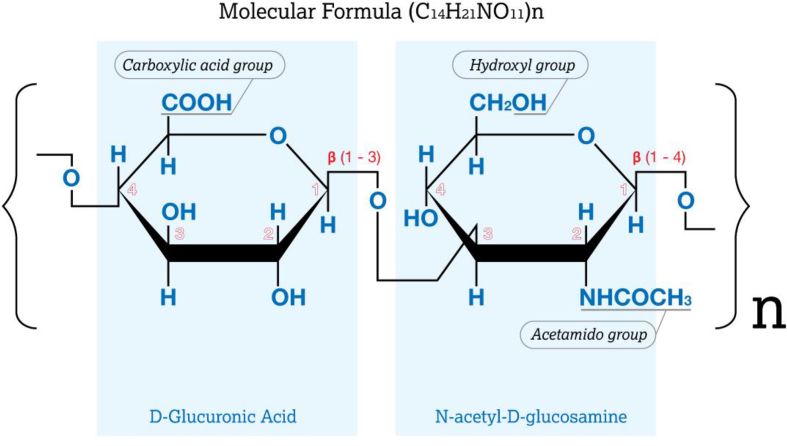
From the chemical standpoint, there are three chemical groups on the HA chain that can be modified: the carboxylic acid group (COOH), hydroxyl group (OH), and the N-acetyl group (CH3CO) which is located on the acetamido group (C2H4NO) [23]. Note that the N-acetyl group contains a methyl (CH3) and carbonyl (C=O) group. Figure 1 shows a diagram of HA and its chemical groups that can be modified.
Figure 1.
Chemical structure of HA showing the chemical groups that can be modified.
Depending on method(s) of modification, the applications of HA has a wide spectrum of possibilities, such as in wrinkle treatment, ophthalmic surgery, drug delivery, wound healing, cartilage, and bone regeneration, etc. [7]. We should draw attention that the HA elasticity and viscosity constitute the practical aspect where their behaviour provides expectation for their performance under physiological conditions, and thus provide the basis to select an appropriate HA product for the clinical treatment. Furthermore, the rheological properties of biological fluids along with the biomechanical properties at the cellular-, tissue- and organ-levels are important to draw an overall understanding of the mechanism by which the entire body system works and can give excellent insights for possible treatments and for related materials. Regarding oral tissues for instance, the elasticity of the dental enamel is in the range of 1030–1646 MPa, whereas it is 1376–1932 MPa for the dental dentin [28]. At the cellular level, human mesenchymal stem cells can differentiate into neurogenic phenotypes if cultured on a hydrogel with viscoelasticity similar to that of the brain (0.1–1.0 kPa), but they differentiate into myogenic phenotypes on hydrogels with viscoelasticity similar to that of the muscles (8–17 kPa). The stem cells of periodontal ligament, dental pulp, and apical papilla can differentiate into osteoblasts, chondrocytes, or neurocytes depending on the nature of trigger and medium in vitro. Thus, the different rheological properties of HA-based gels can trigger different cellular responses [2, 29, 30].
2.5. Considerations for use of biomaterials specific to dentistry
When investigating biomaterials and their medical applications, they are intended to replace, enhance, adjust, mimic or modify a biological function using a synthetic (metal, polymer, etc.) or biological material, either as a standalone material or mixed with other biological, natural or synthetic one. Broadly speaking, biological materials (including HA) used in dental applications are mainly intended to regenerate soft tissues, work as a physical barrier between soft and hard tissues (bone), assist in wound healing and regenerate hard tissues.
Dental procedures related to the regeneration (for soft and hard tissue) and wound healing are broadly divided into two groups: 1) surgical procedures including treatment for sinus lifting, surgical treatment for periodontitis, bone grafting and regeneration, surgical treatment for gingival recession, and socket preservation. 2) non-surgical procedures including treatment for papillae regeneration, non-surgical treatment for periodontitis, mouth ulcers, and gingivitis [31].
For both, surgical and non-surgical procedures, and in relation to regeneration and wound healing, four major factors are needed for optimum outcomes: 1) viable cells as building blocks, 2) the trigger or chemical signal to start and stop the cellular processes, 3) angiogenesis to supply needed nutrients and eliminate cellular waste, and 4) scaffold as a cast for cells to grow in 3D [32]. With regard to these factors, HA (modification dependent) can function as a gel scaffold, trigger, or even as a reservoir for releasing chemical signals (such as hormones or growth factors). HA as a standalone material does not have enough mechanical integrity; therefore, the three possible functions (as gel scaffold, trigger, and reservoir) represent its main applications, as a companion biomaterial to dental procedures, particularly those related to regeneration and wound healing.
Scaffolds (rigid forms and gel forms) should have certain characteristics to enable the cells to adhere to them and grow, and they should be biocompatible and biodegradable. For hydrogel scaffolds (including HA gel scaffolds), their mode of action depends on their physical and biological characteristics [33, 34].
Growth factors are biological molecules which trigger the cells and enzymes during biological processes, but they cannot form a 3D material as they are not structural in their nature. Nevertheless, they can reinforce the basic 3D scaffold. An appropriately designed HA scaffold can work as a reservoir for growth factors, thus containing the cellular triggers and simultaneously releasing the factors over a certain period, depending on its design and on many factors related to the final form and physiochemical properties of the HA molecule as previously mentioned [35]. Generally, a modified cross-linked HA is often first anti-inflammatory in function upon its introduction into a tissue. Upon its degradation, depending on the type and degree of its modification, it may trigger different cellular functions.
From practical and clinical dental treatment viewpoints, there are some other factors to consider regarding the use of biological materials (including HA). Most dental procedures related to regeneration are expected to give basic outcomes (in relation to regeneration time) within 3–6 months of the treatment, and are procedure dependent; therefore, similar or earlier result time frames are expected with the use of biological materials. However, this may not be the case; for instance, if the residence time of a biological material is very short, it may not generate the desired effect. Additionally, it is very common for blood to spread over the treated area what makes its complete isolation impossible. Accordingly, this factor (possibility of mixing with blood) must be considered when designing biological materials for oral applications. If the HA is in the form of a gel scaffold, mixing with blood is considered beneficial as it will possess an intrinsic reservoir of the biological factors contained in the blood. Also, the possible effects between saliva and the biological material should be considered in case of direct contact between them. Finally, one of the most important factors is the continuous existence of bacterial communities, which should be considered carefully upon using biomaterials. Generally, HA have a bacteriostatic effect which manifests by neutralising bacterial hyaluronate lyase enzymes and is applicable with dental implants. Designing HA with a bactericidal agent can be very beneficial for certain clinical cases where high local concentration of the bacterial agent over a certain period is required.
Considering HA as a biological material depends on its final form and characteristics. It can be used as a standalone material to trigger certain cellular functions, a 3D gel scaffold for cells growth and differentiation, gel scaffold working as a physical barrier between soft and hard tissue, gel scaffold containing growth factors or other natural factors (bone morphogenic factors, fibroblast growth factors, platelet-derived growth factors) released over certain time and/or concentrations, carrier for drugs ensuring their sustained release, and co-material with other biological (fibrin, collagen, chondroitin sulphate, hydroxy apatite), natural (cellulose, chitin, starch, alginate, silk), or synthetic materials for enhanced function of either entity. HA applications in dental procedures vary widely, and one should always keep in mind the four essential factors (cell viability, type of cellular trigger, angiogenesis, physicochemical characteristics of the scaffold) required for regeneration or wound healing as evaluation tools when exploring the possible applications or expected outcome results of a natural biomaterial [30, 36].
Some examples on HA uses in dentistry include:
-
•
Injecting HA for papilla regeneration [37].
-
•
Covering the dental implant with HA to enhance its osseointegration.
-
•
Mixing the HA (recommended mixing ratio 1:1) with synthetic bone grafting material for sinus lifting, socket preservation, and periodontal regeneration procedures for enhanced bone growth, even distribution, and increased density of the newly formed bone block [38].
-
•
Covering the surgical area (from inside and outside) by HA to enhance and to accelerate the tissue healing process (including surgical gingival recession treatment) [39].
-
•
Using HA after scaling and root planning as an adjacent therapy for periodontitis.
-
•
Topical application of HA for treatment of oral ulcers.
-
•
Using HA as an adjacent to gingivitis and peri-implantitis treatments.
-
•
Using HA as an adjacent to non-surgical gingival recession treatment.
-
•
Mixing HA with platelet-rich fibrin, plasma, and growth factors to enhance overall outcomes [40].
-
•
Using HA as a nano-sized drug carrier [41].
-
•
Using HA as a matrix to encapsulate stem cells and signalling molecules for reconstruction of temporomandibular joint, salivary glands, dental pulp, dental bone, enamel, root-canal, and mucosa [42, 43, 44].
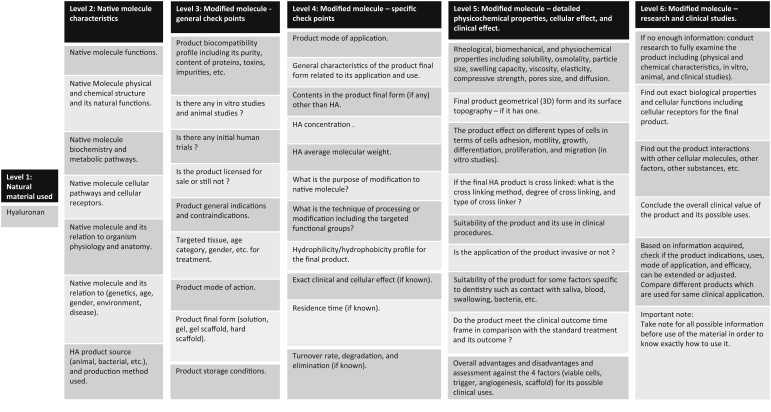
Practically, physicians may need to compare between available HA products to identify differences in terms of their clinical use. Thus, it is logical to provide a model scheme (Figure 2) summarising the relevant considerations for evaluation of an HA product, in anticipation of its possible uses and expected outcomes. This scheme can be used or modified for use with any biological or natural material with generally similar characteristics.
Figure 2.
Scheme of considerations for use of a HA product.
It is worth mentioning that the scheme (Figure 2) has been designed for use by oral care providers as a checklist of different levels depending on available information. Upon knowledge of exact characteristics of the material in use and considering mentioned parameters, it can be bridged with its possible application in dentistry. At the same time, this scheme (Figure 2) can be reversely used in designing a product which matches the desired features of the planned therapy.
3. Conclusion
The biological properties of HA depend on many parameters, and thus it is important for oral care providers to be able to compare between different products. This study provides the scientific background for HA use and a model scheme as a basis to bridge specific characteristics of HA with its potential applications in dental treatments. Comparison of the proposed parameters constitutes a basis for development of new methods, protocols, and products specifically oriented for dentistry.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Stern R., Jedrzejas M. Carbohydrate polymers at the center of life’s origins: the importance of molecular processivity. Chem. Rev. 2008;108:5061–5085. doi: 10.1021/cr078240l. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khateeb R., Prpic J. Hyaluronic acid: the reason for its variety of physiological and biochemical functional properties. Appl. Clin. Res. Clin. Trials Regul. Aff. 2019;6:112–159. [Google Scholar]
- 3.Wight T. Provisional matrix: a role for versican and hyaluronan. Matrix Biol. 2017;60–61:38–56. doi: 10.1016/j.matbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollard T., Earnshaw W., Johnson G., Lippincott-Schwartz J. Cell Biology. third ed. Elsevier Inc.; Philadelphia: 2017. pp. 505–555. [Google Scholar]
- 5.American Chemical Society American chemical society. 2020. https://www.acs.org/content/acs/en.html 2020 Accessed 5 Jan. 2020.
- 6.Pogrel M., Lowe M., Stern R. Hyaluronan (hyaluronic acid) in human saliva. Arch. Oral Biol. 1996;41:667–671. doi: 10.1016/s0003-9969(96)00050-7. [DOI] [PubMed] [Google Scholar]
- 7.Boeriu C., Springer J., Kooy F., van den Broek L., Eggink G. Production methods for hyaluronan. Int. J. Carbohydr. Chem. 2013;2013:1–14. [Google Scholar]
- 8.Lee J. Spicer, Hyaluronan A. A multifunctional, megaDalton, stealth molecule. Curr. Opin. Cell Biol. 2000;12:581–586. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- 9.Ghatak S., Maytin E., Mack J., Hascall V., Atanelishvili I., Moreno Rodriguez R., Markwald R., Misra S. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int. J. Cell Biol. 2015;2015:1–20. doi: 10.1155/2015/834893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser J., Laurent T., Laurent U. Hyaluronan: its nature, distribution, functions and turnover. J. Intern. Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 11.Prajapati V., Maheriya P. 2019. Hyaluronic acid as potential carrier in biomedical and drug delivery applications. Functional Polysaccharides for Biomedical Applications; pp. 213–265. [Google Scholar]
- 12.Dicker K., Gurski L., Pradhan-Bhatt S., Witt R., Farach-Carson M., Jia X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014;10(4):1558–1570. doi: 10.1016/j.actbio.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.J.W K. CRC/Taylor & Francis; Boca Raton: 2006. Practical Aspects of Hyaluronan Based Medical Products; p. 12. [Google Scholar]
- 14.Liu L., Liu Y., Li J., Du G., Chen J. Microbial production of hyaluronic acid: current state, challenges, and perspectives. Microb. Cell Factories. 2011;10:99. doi: 10.1186/1475-2859-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giji S., Arumugam M. Isolation and characterization of hyaluronic acid from marine organisms. Adv. Food Nutr. Res. 2014:61–77. doi: 10.1016/B978-0-12-800269-8.00004-X. [DOI] [PubMed] [Google Scholar]
- 16.Kogan G., Šoltés L., Stern R., Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2006;29:17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 17.Patterson J., Siew R., Herring S., Lin A., Guldberg R., Stayton P. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010;31:6772–6781. doi: 10.1016/j.biomaterials.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnevie E., Galesso D., Secchieri C., Bonassar L. Frictional characterization of injectable hyaluronic acids is more predictive of clinical outcomes than traditional rheological or viscoelastic characterization. PloS One. 2019;14 doi: 10.1371/journal.pone.0216702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kablik J., Monheit G., Yu L., Chang G., Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2009;35:302–312. doi: 10.1111/j.1524-4725.2008.01046.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang G., Huang H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018;25:766–772. doi: 10.1080/10717544.2018.1450910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K., Choi H., Choi E.S., Park M.-H., Ryu J.-H. Hyaluronic acid-coated nanomedicine for targeted cancer therapy. Pharmaceutics. 2019;11:301. doi: 10.3390/pharmaceutics11070301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sionkowska A., Kaczmarek B. Preparation and characterization of composites based on the blends of collagen, chitosan and hyaluronic acid with nano-hydroxyapatite. Int. J. Biol. Macromol. 2017;102:658–666. doi: 10.1016/j.ijbiomac.2017.03.196. [DOI] [PubMed] [Google Scholar]
- 23.Schanté C., Zuber G., Herlin C., Vandamme T. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011;85:469–489. [Google Scholar]
- 24.Bertl K., Bruckmann C., Isberg P., Klinge B., Gotfredsen K., Stavropoulos A. Hyaluronan in non-surgical and surgical periodontal therapy: a systematic review. J. Clin. Periodontol. 2015;42:236–246. doi: 10.1111/jcpe.12371. [DOI] [PubMed] [Google Scholar]
- 25.Eliezer M., Imber J., Sculean A., Pandis N., Teich S. Hyaluronic acid as adjunctive to non-surgical and surgical periodontal therapy: a systematic review and meta-analysis. Clin. Oral Invest. 2019;23:3423–3435. doi: 10.1007/s00784-019-03012-w. [DOI] [PubMed] [Google Scholar]
- 26.Vandana K., Singh S. Use of different concentrations of hyaluronic acid in interdental papillary deficiency treatment: a clinical study. J. Indian Soc. Periodontol. 2019;23:35. doi: 10.4103/jisp.jisp_332_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asparuhova M., Kiryak D., Eliezer M., Mihov D., Sculean A. Activity of two hyaluronan preparations on primary human oral fibroblasts. J. Periodontal. Res. 2018;54:33–45. doi: 10.1111/jre.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chun K., Choi H., Lee J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J. Dent. Biomech. 2014;5 doi: 10.1177/1758736014520809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engler A., Sen S., Sweeney H., Discher D. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Iranparvar A., Nozariasbmarz A., DeGrave S., Tayebi L. Tissue engineering in periodontal regeneration. Appl. Biomed. Eng. Dentistry. 2019:301–327. [Google Scholar]
- 31.Susin C., Wikesjö U. Regenerative periodontal therapy: 30 years of lessons learned and unlearned. Periodontol. 2000;62(2013):232–242. doi: 10.1111/prd.12003. [DOI] [PubMed] [Google Scholar]
- 32.Jabbarzadeh E., Starnes T., Khan Y., Jiang T., Wirtel A., Deng M., Lv Q., Nair L., Doty S., Laurencin C. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: a combined gene therapy-cell transplantation approach. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105(32):11099–11104. doi: 10.1073/pnas.0800069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins M., Birkinshaw C. Hyaluronic acid-based scaffolds for tissue engineering—a review. Carbohydr. Polym. 2013;92:1262–1279. doi: 10.1016/j.carbpol.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Discher D. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 35.Vigier S., Fülöp T. 2016. Exploring the extracellular matrix to create biomaterials. Composition and function of the extracellular matrix in the human body. [Google Scholar]
- 36.Ahmadian E., Dizaj S., Eftekhari A., Dalir E., Vahedi P., Hasanzadeh A., Samiei M. The potential applications of hyaluronic acid hydrogels in biomedicine. Drug Research. 2019;70(1):6–11. doi: 10.1055/a-0991-7585. [DOI] [PubMed] [Google Scholar]
- 37.Awartani F., Tatakis D. Interdental papilla loss: treatment by hyaluronic acid gel injection: a case series. Clin. Oral Invest. 2015;20:1775–1780. doi: 10.1007/s00784-015-1677-z. [DOI] [PubMed] [Google Scholar]
- 38.Cirligeriu L., Cimpean A., Calniceanu H., Vladau M., Sarb S., Raica M., Nica L. Hyaluronic acid/bone substitute complex implanted on chick embryo chorioallantoic membrane induces osteoblastic differentiation and angiogenesis, but not inflammation. Int. J. Mol. Sci. 2018;19:4119. doi: 10.3390/ijms19124119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilloni A., Schmidlin P., Sahrmann P., Sculean A., Rojas M. Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: a randomized controlled clinical trial. Clin. Oral Invest. 2018;23:1133–1141. doi: 10.1007/s00784-018-2537-4. [DOI] [PubMed] [Google Scholar]
- 40.Peck M., Hiss D., Stephen L., Olivier A. The in vitro effect of leukocyte- and platelet-rich fibrin (L-PRF) and cross-linked hyaluronic acid on fibroblast viability and proliferation. S. Afr. Dent. J. 2018;73 [Google Scholar]
- 41.Turcsányi Á., Varga N., Csapó E. Chitosan-modified hyaluronic acid-based nanosized drug carriers. Int. J. Biol. Macromol. 2020;148:218–225. doi: 10.1016/j.ijbiomac.2020.01.118. [DOI] [PubMed] [Google Scholar]
- 42.Ardeshirylajimi A., Golchin A., Vargas J., Tayebi L. Application of stem cell encapsulated hydrogel in dentistry. Appl. Biomed. Eng. Dentistry. 2019:289–300. [Google Scholar]
- 43.Ahmadian E., Eftekhari A., Dizaj S., Sharifi S., Mokhtarpour M., Nasibova A., Khalilov R., Samiei M. The effect of hyaluronic acid hydrogels on dental pulp stem cells behavior. Int. J. Biol. Macromol. 2019;140:245–254. doi: 10.1016/j.ijbiomac.2019.08.119. [DOI] [PubMed] [Google Scholar]
- 44.Lee S., Ryu J., Do M., Namkoong E., Lee H., Park K. NiCHE platform: nature-inspired catechol-conjugated hyaluronic acid environment platform for salivary gland tissue engineering. ACS Appl. Mater. Interfaces. 2020;12(4):4285–4294. doi: 10.1021/acsami.9b20546. [DOI] [PubMed] [Google Scholar]