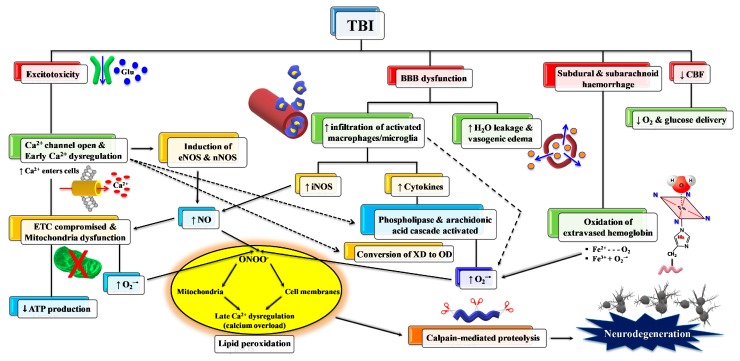
Figure 3.
Schematic representation of some of the main pathological processes characterizing the TBI-associated secondary insult. The force discharged and partly absorbed by the cerebral tissue at the time of impact (primary insult) induces immediate glutamate release by neurons, change in the blood–brain barrier (BBB) permeability, frequent hemorrhage, and decrease in the cerebral blood flow (CBF). Excitotoxic phenomena due to sustained glutamate (Glu) release deeply alter ionic homeostasis, particularly causing an increase in mitochondrial Ca2+. Malfunctioning of the mitochondrial electron transport chain (ETC) and oxidative phosphorylation (OXPHOS) is consequent to increased Ca2+ entry and decreased oxygen and glucose delivery (due to a decrease in CBF), and ultimately generating decreased ATP formation with an energy crisis. Ca2+ also activates endothelial (eNOS) and neuronal (nNOS) isoforms of nitric oxide (NO) synthase, promotes the conversion of xanthine dehydrogenase (XDH) into xanthine oxidase (XO), and triggers the arachidonic acid cascade activating phospholipases. The change in BBB permeability modifies water vascular permeability, causing vasogenic brain edema, and allows infiltration and activation of macrophages/microglia, that are responsible either for NO overproduction by the inducible NO synthase (iNOS) or for the release of pro-inflammatory cytokines. Hematomas generate the release of hemoglobin (Hb) from ruptured erythrocytes and the consequent oxidation of Fe2+ of Hb to Fe3+. This last process, together with XO activity, the arachidonic acid cascade, activated macrophages/microglia, and dysfunctional mitochondria, generates a flow of superoxide anion (O2•−) and gives rise to the reaction with NO and the formation of peroxynitrite (ONOO•−). The damaging action of ROS and RNS on polyunsaturated fatty acids of phospholipids of biological membranes triggers a lipid peroxidation reaction chain, culminating in neuronal cell death.