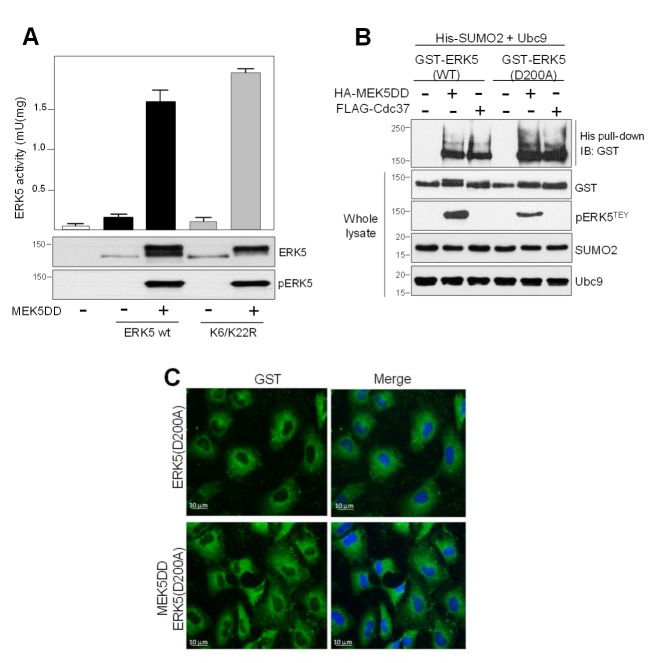
Figure 4.
ERK5 SUMOylation is not necessary for ERK5 kinase activity. (A) HEK293T cells overexpressing GST-tagged ERK5 (wild type or SUMO-deficient K6/22R mutant), alone or in combination with MEK5DD, were lysed, and the GST-ERK5 protein was affinity-purified and assayed for kinase activity, as described in the Methods Section. Activity data are the mean ± SD of two independent experiments, each performed in triplicate. Cell lysates were also immunoblotted for ERK5 (anti-GST antibody) and for phosphorylated ERK5 (pERK5). (B) HEK293T cells were co-transfected with His-tagged SUMO2 and Ubc9, and the indicated combinations of GST-tagged ERK5-D200A (kinase inactive), HA-tagged MEK5DD (constitutively active) and FLAG-tagged Cdc37. After lysing the cells with denaturing buffer containing NEM, SUMOylated ERK5 was affinity-purified using Ni2+-agarose beads and detected by immunoblotting with anti-GST antibody. Levels of overexpressed proteins and phosphorylated ERK5 at the T-loop are shown using the corresponding antibodies. Similar results were obtained in three independent experiments. (C) Immunofluorescence microscopy. PC-3 cells were transfected with GST-tagged ERK5 (wild type or kinase-inactive mutant D200A) and MEK5DD plasmids. After 24 h, cells were fixed with paraformaldehyde and immunofluorescent staining for ERK5 (green) using anti-GST antibody. Nuclei were stained with Hoechst (blue).