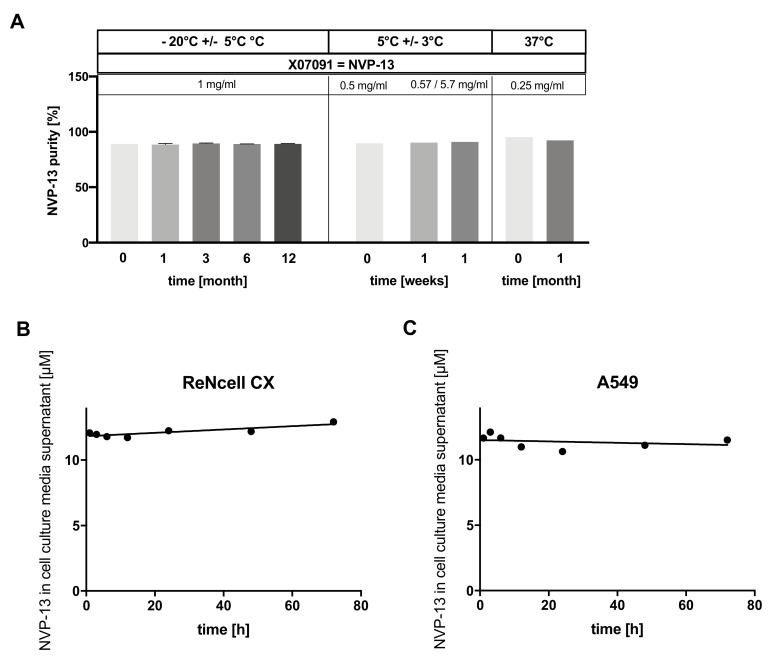
Figure 7.
In-use stability of lead candidate X07091 = NVP-13. (A) Integrity of NVP-13 in 0.9 % NaCl (saline) when incubated with different temperature conditions and for different time intervals. For incubation at −20 °C +/− 5 °C a denaturizing Ion-Pair-Reversed-Pair High Performance Liquid Chromatography (IP-RP-HPLC) with Electrospray-Ionization (ESI)/Mass Spectrometry (MS) was used for determination of relative purity and identity of NVP-13. Aliquots incubated at 5 °C +/− 3 °C were analyzed using an IP-RP-HPLC combined with UV/Mass spectrometry and samples incubated at 37 °C (37 °C as adjusted by the integrated thermostat of New Brunswick Galaxy 170 S Incubator, Eppendorf) were analyzed using IP-RP-UPLC with UV/ESI/MS. Results showed that NVP-13 content was stable under all tested conditions. Discrepancy for content and purity/integrity were within method variability. Values are given as mean and SEM. Determination of intact NVP-13 with a specific bioprobe in the supernatant of cell culture media supernatant of ReNcell CX® (B) and A549 cells (C) after incubation with 10 µM NVP-13 for up to 72 h, n = 3. Line of best fit for (B) and (C) was calculated by a nonlinear fit function.