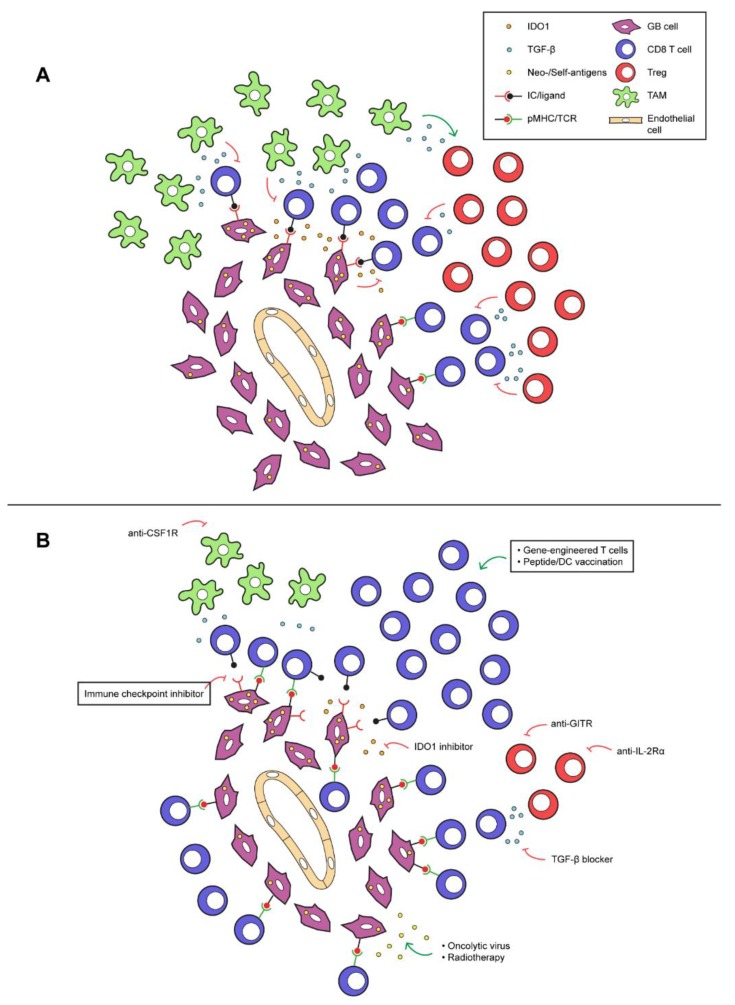
Figure 1.
Exemplary immune-suppressive mechanisms in glioblastoma, and therapeutic interventions to sensitize glioblastoma for T cell treatments. (A) Tumor-associated macrophages (TAMs) and regulatory T cells (Tregs) release immunosuppressive mediators in the glioblastoma microenvironment, such as TGF-β and IL-10 (the latter not depicted). Local production of indoleamine 2,3- dioxygenase (IDO) or tryptophan-2,3-dioxygenase (TDO) by glioblastoma cells depletes tryptophan from the tumor micro-environment, having an adverse effect on the function of CD8 T cells. Also, the immune checkpoint, PD-L1, expressed on glioblastoma cells can engage with PD-1+ T cells to suppress their effector function. (B) Monoclonal antibodies directed against glucocorticoid-induced TNFR related protein (GITR) and IL-2 receptor α (IL-2Rα) may specifically deplete intratumoral Tregs. Immunosuppressive effects exerted by TGF-β can be abolished by small molecule inhibitors, such as galunisertib. IDO and TDO inhibitors (the latter not depicted) may restore tryptophan levels in the micro-environment, causing re-activation of CD8 T cells. TAM-induced immunosuppression may be counteracted using inhibitors against colony-stimulation factor 1 (CSF1) or its receptor. Oncolytic virotherapy and radiotherapy may cause increased release of antigens, which in turn may result in enhanced numbers and activation of intratumoral CD8 T cells. When (one or several of) these interventions are combined with vaccines, immune checkpoint inhibitors (ICIs) or adoptive transfer of effector lymphocytes, this may significantly improve the recruitment and/or activation state of glioblastoma-specific CD8 T cells and lead to restoration of the efficacy of these T cell treatments in glioblastoma.