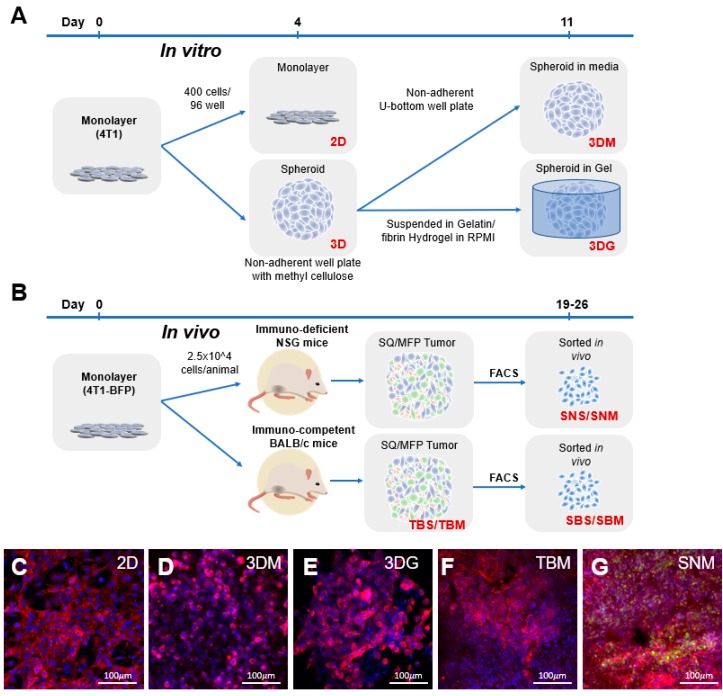
Figure 1.
Experimental overview. (A) The 4T1 in vitro samples originated from low passage number, subconfluent, monolayer cultured cells seeded at 400 cells/well in 96 wells into tissue culture-treated flat-bottom plates or non-adherent U-bottom plates. Following 4 days of culture, monolayer (C) RNA was collected, and spheroids continued to be cultured in wells containing media (D) or cast into a gelatin/fibrin hydrogel (E) for an additional 7 days prior to RNA isolation. (B) In vivo tumor samples were generated by injection of subconfluent, monolayer 4T1-BFP cultures into mammary fat pad (MFP) or subcutaneously into (SQ) back flank locations in immunodeficient (NSG) or BALB/c mice. Tumors were isolated following a 19–26-day growth period yielding tumors ranging from 70 to 140 mm3. RNA samples were processed from bulk (F) or BFP+ cancer cell populations isolated by fluorescently activated cell sorting (FACS) (G). Blue: nuclear DAPI staining; red: phalloidin staining actin filaments; green: BFP expression.