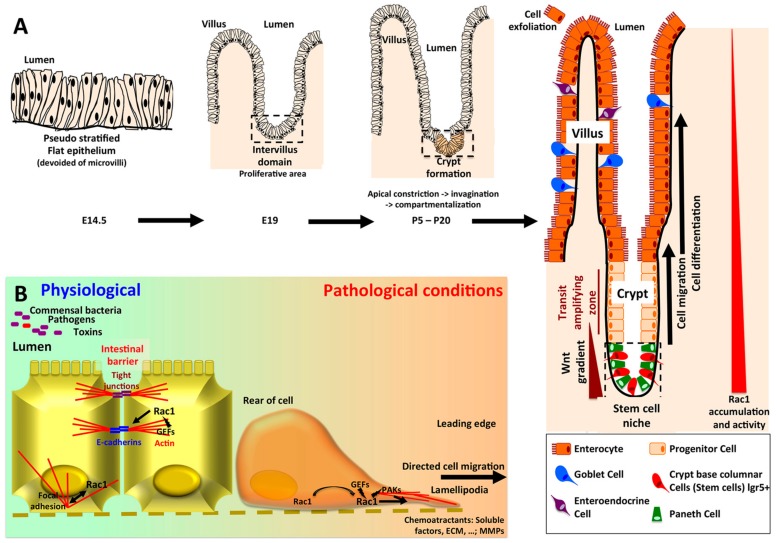
Figure 3.
(A) Ontogeny of the crypt-villus unit. The intestinal epithelium originates from endoderm, whereas the underlying mesenchymal and muscular layers derive from mesoderm. Intestinal ontogenesis follows an anteroposterior axis. In mouse, at embryonic day 14.5, the intestinal epithelium is pseudostratified and subjected to an active proliferation. By embryonic day 18, the villi have emerged in the duodenum and the proliferative area is restricted to the intervillus domain [64]. The crypts began to invaginate by day 5 post partum. First, myosin II-mediated apical constriction allows invagination of the crypt progenitor cells. Subsequently, a hinge region forms between crypts and villi. This process requires Rac1, which locally acts to suppress hemidesmosomal integrins, allowing cell shape changes and the formation of the hinge cells, leading to the compartmentalization of the crypt and villi [50]. In adult intestine, the stem cells -colocalized with Paneth cells in the bottom of the crypt- give rise to progenitor cells which will mainly differentiate into enterocytes, but also in goblet and enteroendocrine cells, and migrate along the villi to exfoliate at the top, whereas downward cell migration and differentiation allows to renew Paneth cells pool [65]. Wnt3 produced by Paneth cells, as well as EGF and Notch stimuli are required for the maintenance of stem cells. In the crypt villus unit, Rac1 expression and activity follow an increasing gradient from the top of the villi to the bottom of the crypts. As regards the colonic epithelium, the main characteristics rely on the absence of villi (flat mucosa), the absence of Paneth cell (substituted by Reg4+ deep crypt secretory cells), and the main type of differentiation being goblet cells [65]. (B) Rac1 in intestinal homeostasis and intestinal barrier repairs. By initiating the formation of E-cadherin complexes, Rac1 plays a critical role in the maintenance of tissue integrity. Upon wound formation, Rac1 localized to the leading edge of the cells, trigger lamellipodia formation through actin cystoskeleton remodeling, and directed cell migration.