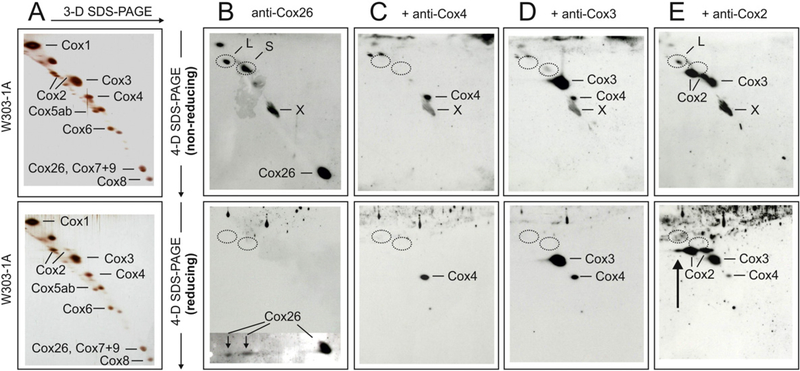
Fig. 6.
Evidence for covalent interaction of Cox26 and Cox2 proteins in isolated complex IV. Gel pieces from 2-D BNE/modified BNE containing complex IV were resolved under non-reducing conditions by 3-D Tricine-SDS-PAGE using 9% acrylamide gels. 3-D gel strips were then incubated under non-reducing (upper panels) or reducing conditions (lower panels) followed by 4-D Tricine-SDS-PAGE using 16% acrylamide gels containing 6 M urea. The 4-D gels were silver-stained (A) or blotted onto PVDF membranes (B-E). Polyclonal anti-Cox26 antibody identified individual Cox26 protein and two bands (L and S) under non-reducing conditions (B, upper panel), and two Cox26 protein spots that were dissociated from bands L and S under reducing conditions (B, lower panel). A local defect of the PVDF membrane (marked X) was also recognized by the antibody. Circles mark the actual or expected positions of bands L and S. (C and D) Anti-Cox4 and anti-Cox3 antibodies that were added consecutively to the same blot membranes (without using stripping protocols) identified individual Cox4 and Cox3 proteins but no bands L and S. Reusing the same blots, an anti-Cox2 antibody finally recognized two forms of individual Cox2 protein and also band L under non-reducing conditions (E, upper panel) in addition to the protein spots recognized before by the anti-Cox4 and anti-Cox3 antibodies. Performing 4-D SDS-PAGE under reducing conditions (E, lower panel), band L was no longer detected but a third Cox2 spot immediately below the expected position ofband L appeared (marked by an arrow).