Short abstract
Objective
To investigate associations between left atrial diameter (LAD) and long-term outcomes in patients with anterior or non-anterior wall ST-elevation myocardial infarction (STEMI).
Methods
Patients with STEMI were included in this secondary analysis of data from a prospective cohort study in which the primary outcome was major adverse cardiovascular event (MACE) occurrence during a 2.5-year follow-up. A LAD cut-off value was obtained through receiver operating characteristic curve analysis. Kaplan-Meier curve and Cox regression analyses were applied. Subgroup Cox regression analysis was also performed, with patients stratified based on left ventricular diastolic diameter (LVEDD, > 55 mm and ≤55 mm). The relationship between LAD and outcomes in patients with anterior or non-anterior wall STEMI was explored using restricted cubic spline functions.
Results
Out of 464 patients, adjusted Cox regression showed that dichotomous (>40 mm) LAD was significantly associated with MACE (hazard ratio 2.978, 95% confidence interval 1.763, 5.030) in patients with anterior wall but not non-anterior wall STEMI. The association was not different between normal and enlarged LVEDD groups.
Conclusions
A left atrium > 40 mm may indicate higher risk of MACE in patients with anterior wall STEMI, even in patients with normal left ventricular structure. This relationship was not observed in patients with non-anterior wall STEMI.
Keywords: Left atrial size, anterior wall myocardial infarction, echocardiography
Introduction
ST-elevation myocardial infarction (STEMI) is a leading life-threatening disease worldwide.1 Although primary percutaneous coronary intervention has been approved as the gold standard therapy for patients with STEMI over the past 20 years,2–4 patients with anterior wall STEMI have a worse prognosis than those with non-anterior wall STEMI.5 Therefore, developing prognostic markers for patients with STEMI is essential to enable clinicians to develop risk stratifications for these patients. Such risk stratification strategies may improve patient management and lead to improved clinical outcomes in patients with STEMI.
Left atrium (LA) structural and functional remodelling may reflect the chronic volume or pressure overload of LA and left ventricle (LV) diastolic dysfunction as a result of chronically elevated LV filling pressure.6–8 Awareness has grown regarding the importance of echocardiographic parameters measuring LA size and function, and these parameters have been developed into predictive markers of clinical cardiovascular risk, such as LA expansion index, LA emptying fraction, LA functional index and LA diameter (LAD).6,7,9 In addition, two prospective cohort studies reported that left atrial function parameters could predict atrial fibrillation and major adverse cardiovascular events (MACEs) in patients with STEMI.8,10 LAD, as a more realistic indicator of LA size, has been shown to be related to stroke, cognitive function, death, and multiple cardiovascular events in both the general and hypertensive populations.11–17 However, there is little information available on the impact of LAD on patients with STEMI. Although LAD may not represent an accurate picture of LA size, it reflects the real clinical scenario, because most hospitals use two-dimensional echocardiography and LA volume is not readily measured in China. Furthermore, anterior wall STEMI is the most serious type of STEMI, and data regarding the predictors of long-term prognosis in patients with anterior wall STEMI are sparse.18 The effect of LAD on clinical outcomes may vary between patients with anterior versus non-anterior wall STEMI. Therefore, the present study sought to determine whether LAD was associated with the long-term incidence of any MACE in patients with anterior or non-anterior wall STEMI, and whether this association remains in patients with normal left ventricular structure.
Patients and methods
Study population
The present secondary analyses included data from a population of patients with STEMI who were included in a previously published prospective cohort study, and whose data were made available via the Dryad public database.19,20 Thus, data for this secondary analyses were extracted from the Dryad Digital Repository (https://datadryad.org/stash/dataset/doi:10.5061/dryad.pf56m). The study was approved by the ethics committee for medical research at the First Affiliated Hospital of Xi’an Jiaotong University, and because anonymized patient data were used, the requirement for written informed consent was waived by the review board. Inclusion criteria for the present study comprised patients who had been diagnosed with STEMI and who had received primary percutaneous coronary intervention treatment. Exclusion criteria comprised the following: (1) previous cardiovascular diseases, including ventricular fibrillation, untreated third or advanced degree of atrioventricular block, cardiogenic shock, or secondary hypertension; (2) severe hepatic diseases, renal disease, endocrine diseases, or cerebrovascular attack; (3) known contraindication to statins, heparin, aspirin, clopidogrel, contrast, or glycoprotein IIb/IIIa inhibitor; (4) diagnosis of malignancy; (5) major surgery or trauma within 6 weeks of original study inclusion; or (6) incomplete clinical data. The original prospective cohort study included 464 patients with STEMI who were admitted to the First People’s Hospital of Taizhou, Zhejiang, China between January 2010 and October 2014, and had received primary percutaneous coronary intervention treatment.19 All of the patients with STEMI had been admitted to the emergency department within 12 h of symptom onset, and prior to surgery, patients had been administered 300 mg oral aspirin, 300 mg clopidogrel, and 10 000 international units of heparin (additional dosage during the operation). Patients were administered glycoprotein IIb/IIIa inhibitor when they exhibited a high thrombotic burden. STEMI was diagnosed according to symptoms of myocardial ischaemia, such as notable chest pain or discomfort that lasted longer than 30 min, and dynamic electrocardiogram changes including ST segment elevation in two or more continuous leads or a newly developed left bundle branch block.
Data collection
The following data were extracted from the database: (1) Basic characteristics that had been collected at hospital admission, comprising age, sex, heart rate, systolic blood pressure, anterior wall myocardial infarction, Killip’s Classification > Class I, previous myocardial infarction, hypertension and diabetes mellitus; (2) Data regarding laboratory test parameters from examinations performed within 48 h of hospital admission, including total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, fasting blood glucose, creatinine, uric acid, peak cardiac troponin-I, D-Dimer, and peak creatine kinase-MB; (3) Echocardiographic and electrocardiographic data, including left ventricular diastolic diameter (LVEDD), LAD and pathological Q-wave. Doppler and two-dimensional echocardiography was conducted on every patient within five days following primary percutaneous coronary intervention, according to assessment standards of the American Society of Echocardiography.19,21 In order to avoid measurement errors, measurements were conducted three times to get the average value for patients with atrial fibrillation, and twice for patients in sinus rhythm. The primary outcome in the original study was MACE,19 which is a composite generated from the presence of any clinically driven target lesion revascularization, recurrent myocardial infarction of the target vessel, congestive heart failure, and cardiac death. All patients were visited to observe any MACE occurrence following primary percutaneous coronary intervention, for up to 30 months after the initial hospital admission.
Statistical analyses
Patients were classified into two groups: anterior wall STEMI or non-anterior wall STEMI, based on clinical diagnosis. The optimal cut-off for LAD (40 mm) was defined using analysis of a receiver operating characteristic (ROC) curve to achieve the highest Youden index. Normally distributed, continuous parameters are presented as mean ± SD and were analysed for between-group differences using Student’s t-test. Data without normal distribution are presented as median (interquartile range) and were compared using Mann–Whitney U-test. Categorical variables are presented as n (%) prevalence and between-group differences were analysed using χ2-test.
The relationship between the LAD and MACE was analysed using Cox proportional hazards model in patients with anterior and non-anterior wall STEMI and in LVEDD subgroups (LVEDD > 55 mm and LVEDD ≤ 55 mm) after controlling for potential confounders (age, sex, diabetes, total cholesterol, high-density lipoprotein cholesterol, systolic pressure, previous myocardial infarction, anterior wall myocardial infarction, Gensini score, LVEDD, pathological Q wave and Killip’s classification > Class I). In China, the normal range for LVEDD has a maximum value of 55 mm. Kaplan-Meier cumulative survival curves were stratified by LAD > 40 mm and the difference between the two curves was analysed using a log-rank test. Multivariable Cox regression with restricted cubic splines was used to test whether the relationship between LAD and outcomes was linear in patients with anterior and non-anterior wall STEMI. The reference point for LAD was 40 mm. Four knots were defined as the 5th, 35th, 65th, 95th percentiles of LAD distribution in the two groups. Two-sided P values < 0.05 were considered statistically significant. All statistical analyses were conducted with R 3.5.1 software (https://www.r-project.org/) and SPSS for Windows, version 24.0 (SPSS Inc, Chicago, IL, USA).
Results
Basic characteristics
In the original study, 572 patients with STEMI were initially admitted to the First People’s Hospital of Taizhou and underwent primary percutaneous coronary intervention.19 Of these, 108 patients did not meet the eligibility criteria, thus, data from a total of 231 patients with anterior wall STEMI and 233 patients with non-anterior wall STEMI were included in the present study (Table 1). In the anterior and non-anterior wall STEMI groups, respectively, there were 174 (75.3%) and 181 (77.7%) male patients, and mean age was 61.6 ± 12.5 and 64.4 ± 11.1 years. In patients with anterior wall STEMI, 68 (29.4%) had a left atrium larger than 40 mm, and all parameters were similar between patients with an enlarged LA (LAD > 40 mm) and those with a normal LA (LAD ≤ 40 mm; all P > 0.05). In patients with non-anterior wall STEMI, 73 (31.3%) had a left atrium larger than 40 mm. Triglyceride and fasting blood glucose levels were lower in patients with an enlarged LA, however, D-dimer levels were higher (P < 0.05; Table 1). Echocardiographic, electrocardiographic, and angiographic characteristics are listed in Table 2. In patients with anterior wall STEMI, median LVEDDs were 55 mm in the enlarged LA group and 49 mm in the normal LA group (P < 0.001). Among patients with non-anterior wall STEMI, median LVEDD values in the enlarged and normal LA groups were 53 mm and 49 mm, respectively (P = 0.005). No other statistically significant between-group differences were detected.
Table 1.
Basic demographic and clinical characteristics, and laboratory parameters, in patients with anterior wall ST-elevation myocardial infarction (STEMI) or non-anterior wall STEMI.
|
Patients with anterior wall STEMI |
||||
|---|---|---|---|---|
| Variable | Whole group n = 231 | LAD > 40 mm n = 68 | LAD ≤ 40 mm n = 163 | Statistical significance |
| Basic characteristics | ||||
| Age, years | 61.6 ± 12.5 | 62.5 ± 12.4 | 61.2 ± 12.6 | NS |
| Male | 174 (75.3%) | 57 (83.8%) | 117 (71.8%) | NS |
| Hypertension | 131 (56.7%) | 41 (60.3%) | 90 (55.2%) | NS |
| Diabetes mellitus | 85 (36.8%) | 25 (36.8%) | 60 (36.8%) | NS |
| Previous MI | 21 (9.1%) | 4 (5.9%) | 17 (10.4%) | NS |
| Killip’s classification > I | 68 (29.4%) | 16 (23.5%) | 52 (31.9%) | NS |
| Systolic blood pressure, mmHg | 133.6 ± 27.1 | 136.7 ± 28.2 | 132.4 ± 26.5 | NS |
| Heart rate, beats/min | 80 [67–93] | 84 [72–94.5] | 77 [66–92] | NS |
| Laboratory test | ||||
| TC, mmol/l | 5.6 ± 1.1 | 5.6 ± 1 | 5.6 ± 1.2 | NS |
| TG, mmol/l | 1 [0.5–1.5] | 1.1 [0.6–1.5] | 0.9 [0.5–1.4] | NS |
| HDL-C, mmol/l | 1.2 [1–1.5] | 1.3 ± 0.3 | 1.2 ± 0.3 | NS |
| LDL-C, mmol/l | 3 [2.5–3.6] | 3.1 [2.7–3.7] | 2.9 [2.4–3.6] | NS |
| Fasting blood glucose, mmol/l | 7.5 [6–9.9] | 7.5 [6.1–10.1] | 7.6 [5.9–9.8] | NS |
| Creatinine, mmol/l | 76 [60–86] | 77.1 [56.5–90.1] | 75.3 [60–86] | NS |
| Uric acid, mmol/l | 332 [284–389] | 342.5 [291–382.3] | 331.5 [281.7–390] | NS |
| Peak cTnI, ng/ml | 13.1 [3.9–26.2] | 11.9 [3.2–26.4] | 14.1 [4.1–26.1] | NS |
| D-Dimer, mg/l | 0.9 [0.2–1.7] | 1 [0.1–1.7] | 0.9 [0.3–1.6] | NS |
| Peak CK-MB, U/l | 106 [49–194] | 105 [40.5–211] | 107 [50–191] | NS |
|
Patients with non-anterior wall STEMI |
||||
|
Variable |
Whole group n = 233 |
LAD > 40 mm n = 73 |
LAD ≤ 40 mm n = 160 |
Statistical significance |
| Age, years | 64.4 ± 11.1 | 63.7 ± 11.4 | 64.7 ± 11 | NS |
| Male | 181 (77.7%) | 57 (78.1%) | 124 (77.5%) | NS |
| Hypertension | 134 (57.5%) | 36 (49.3%) | 98 (61.3%) | NS |
| Diabetes mellitus | 65 (27.9%) | 20 (27.4%) | 45 (28.1%) | NS |
| Previous MI | 34 (14.6%) | 12 (16.4%) | 22 (13.8%) | NS |
| Killip’s classification>I | 44 (18.9%) | 16 (21.9%) | 28 (17.5%) | NS |
| Systolic blood pressure, mmHg | 129.9 ± 27.2 | 128 ± 25.9 | 130.8 ± 27.8 | NS |
| Heart rate, beats/min | 72.6 ± 16.1 | 71 [60–87] | 71.5 [62.5–82] | NS |
| Laboratory test | ||||
| TC, mmol/l | 5.7 ± 1.1 | 5.8 ± 1.3 | 5.6 ± 1 | NS |
| TG, mmol/l | 1.2 ± 0.9 | 0.8 [0.6–1.4] | 1.1 [0.6–1.7] | P = 0.039 |
| HDL-C, mmol/l | 1.2 ± 0.2 | 1.1 ± 0.3 | 1.2 ± 0.2 | NS |
| LDL-C, mmol/l | 3 ± 0.7 | 3.1 [2.6–3.7] | 3 [2.4–3.5] | NS |
| Fasting blood glucose, mmol/l | 7.5 ± 2.6 | 6.4 [5–8.4] | 7.2 [5.8–9.7] | P = 0.028 |
| Creatinine, mmol/l | 75.6 ± 27.4 | 72 [63–87] | 74.4 [63.2–83] | NS |
| Uric acid, mmol/l | 339.6 ± 73.1 | 325.5 [290–409] | 340.5 [270–396.5] | NS |
| Peak cTnI, ng/ml | 17.1 ± 13 | 11.6 [4.9–29] | 16.5 [5–30.2] | NS |
| D-Dimer, mg/l | 1 ± 0.8 | 1.1 [0.4–2] | 0.7 [0.2–1.6] | P = 0.036 |
| Peak CK-MB, U/l | 125.9 ± 87.9 | 104 [57–196] | 105 [38–192.5] | NS |
Categorical parameters are presented as frequency (%); Continuous parameters are presented as mean ± SD or median [interquartile range].
LAD, left atrial diameter; MI, myocardial infarction; WBC, white blood cells; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; cTnI, cardiac troponin I; CK-MB, creatine kinase-myocardial band.
NS, no statistically significant between-group difference (P > 0.05; χ2-test, Student’s t-test or Mann–Whitney U-test, as appropriate).
Table 2.
Echocardiographic, electrocardiographic and angiographic characteristics and clinical outcome in patients with anterior or non-anterior wall STEMI, grouped according to size of left atrial diameter.
|
Patients with anterior wall STEMI |
|||
|---|---|---|---|
| Variable | LAD > 40 mm n = 68 | LAD ≤ 40 mm n = 163 | Statistical significance |
| LVEDD, mm | 55 [48–58] | 49 [45–54] | P < 0.001 |
| LAD, mm | 43 [42–43.5] | 35 [31–38] | P < 0.001 |
| Pathological Q-wave | 30 (44.1%) | 82 (50.3%) | NS |
| Gensini score | 79.5 [49.5–99] | 68 [38–102] | NS |
| Number of stents, n (%) | NS | ||
| One | 48 (68.8%) | 114 (69.9%) | |
| Two | 18 (26.5%) | 42 (25.8%) | |
| Three | 2 (2.9%) | 7 (4.3%) | |
| Culprit vessel, n (%) | NS | ||
| Left anterior descending branch | 50 (73.5%) | 115 (70.6%) | |
| Left circumflex coronary artery | 8 (11.7%) | 19 (11.7%) | |
| Right coronary artery | 10 (14.7%) | 29 (17.8%) | |
|
Patients with non-anterior wall STEMI |
|||
|
Variable |
LAD > 40 mm n = 73 |
LAD ≤ 40 mm n = 160 |
Statistical significance |
| LVEDD, mm | 53 [45–57] | 49 [45–53] | P = 0.005 |
| LAD, mm | 44 [43–46] | 36 [32–38] | P < 0.001 |
| Pathological Q-wave | 34 (46.6%) | 77 (48.1%) | NS |
| Gensini score | 85 [53–105] | 67 [35.5–99] | NS |
| Number of stents, n (%) | NS | ||
| One | 49 (67.1%) | 91 (56.9%) | |
| Two | 24 (32.9%) | 64 (40%) | |
| Three | 0 (0%) | 5 (3.1%) | |
| Culprit vessel, n (%) | NS | ||
| Left anterior descending branch | 21 (28.8%) | 47 (29.4%) | |
| Left circumflex coronary artery | 14 (19.2%) | 31 (19.4%) | |
| Right coronary artery | 38 (52.0%) | 82 (51.3%) | |
Data presented as median [interquartile range] or n (%) prevalence.
LVEDD, left ventricular and diastolic diameter; LAD, left atrial diameter; STEMI, ST-elevation myocardial infarction.
NS, no statistically significant between-group difference (P > 0.05; χ2-test or Mann–Whitney U-test, as appropriate).
Relationship between LAD and MACE
The cut-off value for LAD that was predictive of MACE was evaluated by ROC analysis. With a LAD cut-off of 40 mm, for differentiating between patients with or without MACE, the area under the curve was 0.580 (95% confidence interval [CI] 0.521, 0.639). The negative predictive value was 78.9% and the positive predictive value was 35.5%, with a specificity of 73.7%, and sensitivity of 42.4%.
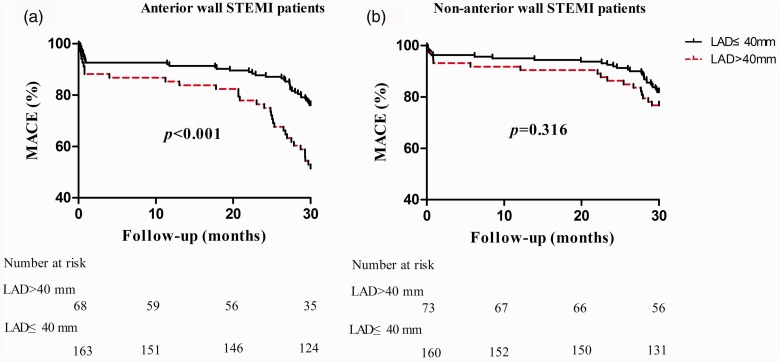
The risk of experiencing MACE differed significantly according to whether LAD was above or below 40 mm, as shown in the Kaplan–Meier plot for patients with anterior wall STEMI (P < 0.001) but not for patients with non-anterior wall STEMI (P = 0.316, Figure 1). Cox regression analyses of the association between LAD and MACE are shown in Table 3. A total of 72 patients (31.2%) with anterior wall STEMI and 46 patients (19.7%) with non-anterior wall STEMI experienced MACEs. In patients with anterior wall STEMI, unadjusted Cox regression analysis showed a significant association between LAD (>40 mm) and long-term MACE (hazard ratio [HR] 2.404, 95% CI 1.511, 3.825; P < 0.001). This statistically significant relationship remained in age- and multivariate-adjusted Cox regression models (age-adjusted, HR 2.266, 95% CI 1.422, 3.612, P < 0.001; and multivariate-adjusted, HR 2.978, 95% CI 1.763, 5.030, P < 0.001). However, no statistically significant relationship was observed between LAD and MACE in patients with non-anterior wall STEMI (Table 3). Using a multivariate-adjusted Cox regression model, age, total cholesterol, pathological Q wave and Killip’s classification > Class I were found to be associated with MACE in patients with anterior wall STEMI, and age and previous myocardial infarction were associated with MACE in patients with non-anterior wall STEMI (interaction P value = 0.045; Table 3).
Figure 1.
Kaplan-Meier curves of MACE incidence during 2.5-years of follow-up in patients with (a) anterior wall STEMI or (b) non-anterior wall STEMI, categorized according to LAD. MACE, major adverse cardiovascular event; LAD, left atrial diameter; STEMI, ST-elevation myocardial infarction.
Table 3.
Cox regression models for the association between LAD (>40 mm) and MACE in patients with anterior wall or non-anterior wall STEMI.
| Model | No. of cases | HR (95% CI) | Statistical significance | Interaction significance |
|---|---|---|---|---|
| Unadjusted | NS | |||
| Total-population | 118 (25.4) | 1.869 (1.297, 2.693) | P < 0.001 | |
| Anterior wall STEMI | 72 (31.2) | 2.404 (1.511, 3.825) | P < 0.001 | |
| Non-anterior wall STEMI | 46 (19.7) | 1.357 (0.746, 2.470) | NS | |
| Age-adjusted | NS | |||
| Total-population | 118 (25.4) | 1.830 (1.270, 2.638) | P = 0.001 | |
| Anterior wall STEMI | 72 (31.2) | 2.266 (1.422, 3.612) | P <0.001 | |
| Non-anterior wall STEMI | 46 (19.7) | 1.397 (0.768, 2.543) | NS | |
| Multivariate-adjusted* | P = 0.045 | |||
| Total-population | 118 (25.4) | 1.859 (1.259, 2.744) | P = 0.002 | |
| Anterior wall STEMI | 72 (31.2) | 2.978 (1.763, 5.030) | P < 0.001 | |
| Non-anterior wall STEMI | 46 (19.7) | 1.166 (0.959, 2.288) | NS |
Data presented as n (%) prevalence.
*adjusted for sex, age, systolic pressure, diabetes mellitus, previous MI, anterior wall MI, high-density lipoprotein cholesterol, total cholesterol, Gensini score, LVEDD, Killip’s classification > Class I and pathological Q wave.
LAD, left atrial diameter; MACE, major adverse cardiovascular event; STEMI, ST-elevation myocardial infarction; HR, hazard ratio; CI, confidence interval; MI, myocardial infarction; LVEDD, left ventricular diastolic diameter.
NS, no statistically significant association (P > 0.05).
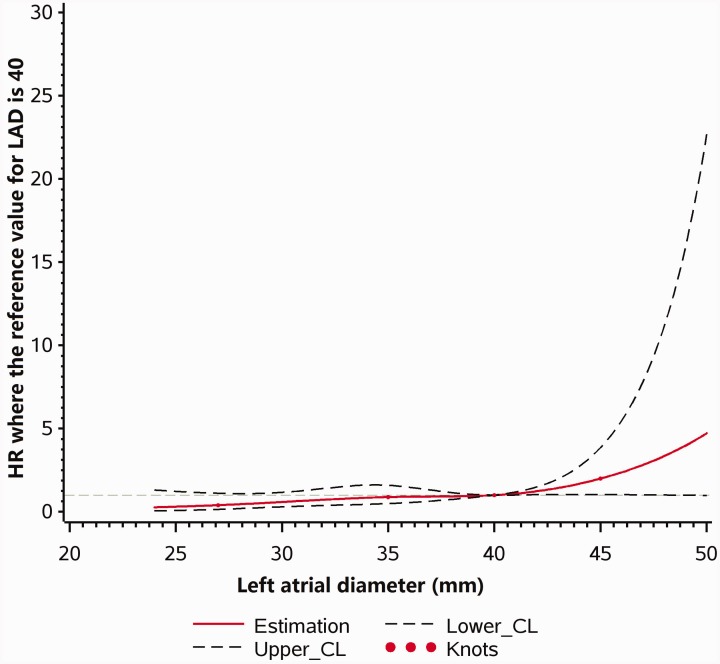
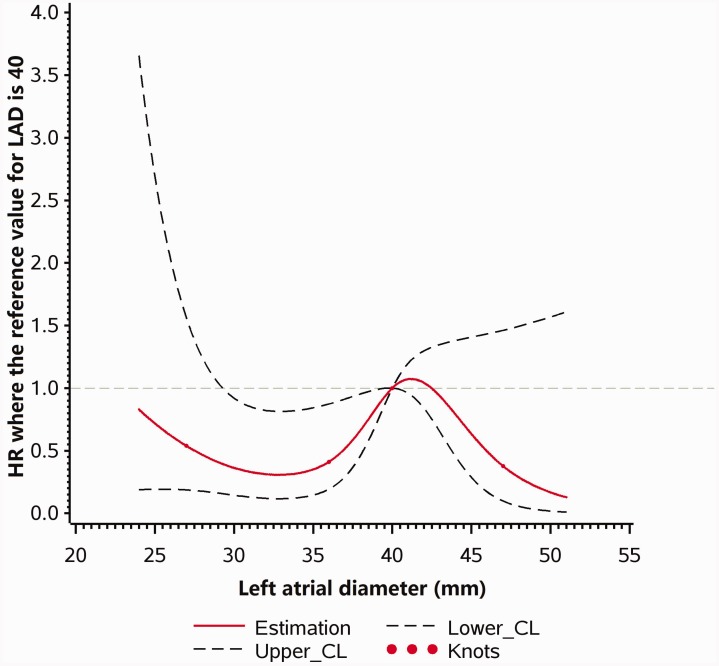
Multivariable Cox regression models with restricted cubic splines are shown in Figures 2 and 3. As LAD increased, the risk of MACE increased in patients with anterior wall STEMI (P linearity = 0.01, P nonlinearity = 0.55). However, no statistically significant relationship was found between LAD and MACE in patients with non-anterior wall STEMI (P linearity = 0.13, P nonlinearity = 0.08).
Figure 2.
Multivariable Cox regression models with restricted cubic splines, showing adjusted HRs for MACE according to LAD in patients with anterior wall STEMI. MACE, major adverse cardiovascular event; HR, hazard ratio; LAD, left atrial diameter; STEMI, ST-elevation myocardial infarction; CL, confidence limit.
Figure 3.
Multivariable Cox regression models with restricted cubic splines, showing adjusted HRs for MACE according to LAD in patients with non-anterior wall STEMI. MACE, major adverse cardiovascular event; HR, hazard ratio; LAD, left atrial diameter; STEMI, ST-elevation myocardial infarction; CL, confidence limit.
Stratification according to normal or enlarged LVEDD
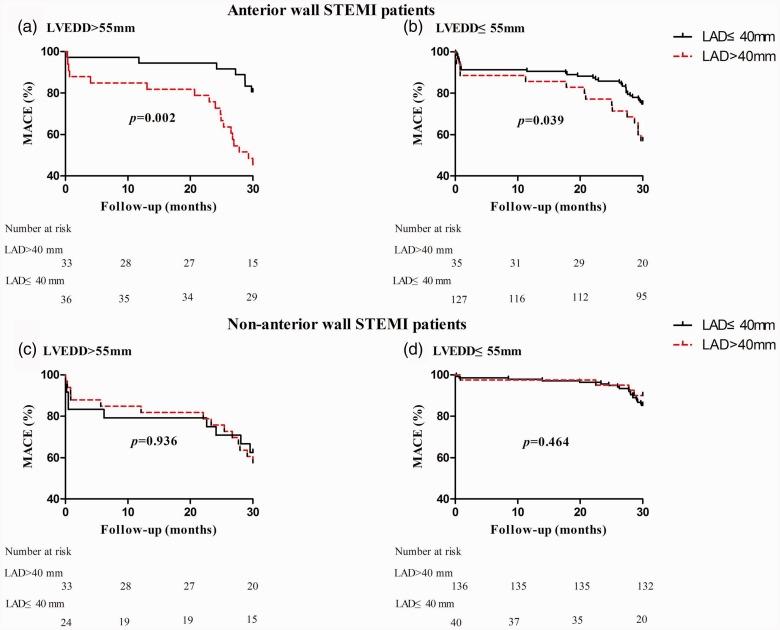
Kaplan–Meier survival curves in LVEDD subgroups (LVEDD > 55 mm versus LVEDD ≤ 55 mm) are shown in Figure 4. In patients with anterior wall STEMI, LAD > 40 mm was predictive of MACE in both of the LVEDD subgroups (P = 0.002 and P = 0.039, LVEDD > 55 mm and ≤ 55 mm, respectively). In patients with non-anterior wall STEMI, the analysis revealed no statistically significant association between LAD and MACE risk in either the normal or enlarged LVEDD subgroups (P = 0.464 and P = 0.936, respectively). Multivariable analyses in the LVEDD subgroups are listed in Table 4. In patients with anterior wall STEMI, the association between LAD (>40 mm) and MACE was evident in both the normal LVEDD group (HR 2.350, 95% CI 1.176, 4.697, P = 0.016) and the enlarged LVEDD group (HR 3.954, 95% CI 1.378, 11.348, P = 0.011). However, no statistically significant relationship was observed between LAD and MACE in patients with non-anterior wall STEMI, regardless of LVEDD. No statistically significant interactions were found in patients with anterior or non-anterior wall STEMI (P = 0.082 and P = 0.721).
Figure 4.
Prognostic effect of LAD on MACE during a 2.5-year follow-up in patients with either (a) anterior wall STEMI and LVEDD > 55 mm, (b) anterior wall STEMI and LVEDD ≤ 55 mm, (c) non-anterior wall STEMI and LVEDD > 55 mm, or (d) non-anterior wall STEMI and LEVDD ≤ 55 mm. MACE, major adverse cardiovascular event; LAD, left atrial diameter; STEMI, ST-elevation myocardial infarction; LVEDD, left ventricular diastolic diameter.
Table 4.
Cox regression models for the association between LAD and MACE in patients with anterior wall or non-anterior wall STEMI, sub-grouped according to LVEDD.
|
LAD > 40mm |
||||
|---|---|---|---|---|
| Subgroup* | No. of cases | HR (95% CI) | Statistical significance | Interaction significance |
| Anterior wall STEMI | NS | |||
| LVEDD > 55 | 25 (36.2) | 3.954 (1.378, 11.348) | P = 0.011 | |
| LVEDD ≤ 55 | 47 (29.0) | 2.350 (1.176, 4.697) | P = 0.016 | |
| Non-anterior wall STEMI | NS | |||
| LVEDD > 55 | 22 (38.6) | 1.156 (0.356, 3.752) | NS | |
| LVEDD ≤ 55 | 24 (13.6) | 0.772 (0.236, 2.530) | NS | |
Data presented as n (%) prevalence.
*Models adjusted for sex, age, systolic pressure, diabetes mellitus, previous MI, anterior wall MI, high-density lipoprotein cholesterol, total cholesterol, Gensini score, Killip’s classification > Class I and pathological Q wave.
LAD, left atrial diameter; MACE, major adverse cardiovascular event; STEMI, ST-elevation myocardial infarction; LVEDD, left ventricular diastolic diameter; MI, myocardial infarction; HR, hazard ratio; CI, confidence interval.
NS, no statistically significant association (P > 0.05).
Discussion
The results of the present study suggest that a large LA may have increased the risk of MACE during a 2.5-year follow-up period in patients with anterior wall STEMI, but not in patients with non-anterior wall STEMI, after adjusting for multiple variables. Subgroup analyses further suggested that the association between LAD and 2.5-year outcomes in patients with anterior wall STEMI did not vary with LVEDD structure (>55 mm or ≤55 mm).
Prior to the present study, LAD has been thought of as a predictor of cardio-cerebrovascular events, such as atrial fibrillation, heart failure, stroke, death, and other cardiovascular diseases in the general or hypertensive populations.9,13–17,22 In addition, LAD has been shown to correlate with endogenous secretion of atrial natriuretic peptide in patients with mitral stenosis,23 and cognitive function in older adults.11 However, none of these previously published studies compared patients with anterior and non-anterior wall STEMI regarding the relationship between LAD and patient prognosis. To the best of the authors’ knowledge, the present study is the first to investigate the association between LAD and prognosis in patients with anterior or non-anterior wall STEMI, and to explore whether this association is altered with LVEDD.
The possible mechanism for this relationship in patients with anterior wall STEMI may be explained as follows: First, LA dilatation and stretch of the atrial myocardium is caused by increased atrial wall tension in response to the chronic increase of filling pressure of the LV.8,13,24 In long-term deterioration of the LV diastolic function, the LA function is also impaired; Secondly, compared with LV myocytes, LA myocytes are more sensitive and react quickly when they receive pathological stimulation. The structural remodelling of the LA due to LA enlargement may lead to altered myocardial energy production and utilization, higher fibrosis in myocytes, and disordered electrical activity and neurohormonal remodelling.25,26 Consequently, the series of changes mentioned previously may cause atrial fibrillation, heart failure, and related adverse cardiovascular outcomes. Furthermore, atrial fibrillation could cause atrial remodelling and persistent maintenance of atrial fibrillation;26 Thirdly, static blood flow and thrombus formation, that occurs when LA size increases disproportionately, increases the possibility of thrombus formation and stroke risk;17,22 Finally, LAD may be associated with 2.5-year outcomes in patients with anterior wall STEMI, independent of LVEDD structure, due to the fact that LAD was dichotomized by a value of 40 mm, which was consistent with cut-off values reported in previously published studies. This indicates that 40 mm is a common LA warning value for predicting cardiovascular outcomes in research conclusions.12,24,27,28
The present study did not find an association between LAD and MACE in patients with non-anterior wall STEMI. The exact reason for this result is unknown and requires further investigation. It is conceivable that anatomical, architectural, and physiological differences may lead to disparities in the response to ischaemic injury among the different infarct sites of STEMI.29 Accordingly, these factors may cause different prognoses between anterior wall STEMI and non-anterior wall STEMI. The left anterior descending coronary artery is the most likely site of occlusion and ischaemia in patients with anterior wall STEMI, and an occlusion of this artery may cause greater LV dysfunction and resultant LA remodelling.18,30 Therefore, it may be easier to observe the correlation between an enlarged LAD and adverse outcomes in patients with anterior wall STEMI than in patients with non-anterior wall STEMI. Furthermore, regional dyskinesis may be easily observed in the anterior wall because of anatomic-mechanical factors. Anterior wall STEMI may exert greater transmural dys-synergic force against the injured anterior wall than the inferior-posterior wall. Moreover, the pressure and resistance of the left coronary arterial system are higher than other coronary arteries. Acute and chronic collaterals are less likely to form from the left anterior descending coronary artery, and this may influence downstream perfusion.29,31
Several limitations of the present study should be considered. First, the individual components of MACE were not available from the Dryad Digital Repository, therefore, the association between LAD and a single outcome could not be analysed. In spite of this, MACE is the most commonly used composite end point in cardiovascular research, particularly regarding complications related to primary percutaneous coronary intervention. Secondly, the relatively small sample size in the study may not provide sufficient power in order to detect the association between LAD and MACE in patients with non-anterior wall STEMI, and the interplay between LVEDD and LAD on MACE. Thirdly, although many confounders were already adjusted in the present multivariable analysis when analysing the association between LAD and MACE, other potential clinical confounding factors may have also affected the prognosis in patients with STEMI who underwent primary percutaneous coronary intervention, such as medication treatment during hospital and discharge. Finally, a previous study reported that the indexed LA volume was a sensitive predictor of future cardiovascular events rather than LA diameter for patients in sinus rhythm.24 Another study found that increased LA volume, acquired within the first 48 h of admission, was associated with five-year mortality in patients with acute myocardial infarction.32 Furthermore, the LA volume and LA volume index were not obtained because only two-dimensional echocardiography was performed in the study population included in the present study. Whether or not the role of LA volume and LA volume index in predicting cardiovascular events is superior to LAD was not determined in the study. Further research with a larger sample size is needed to evaluate this problem. Nonetheless, as a more direct and less time-consuming parameter of measuring LA size, LAD may hold greater appeal for predicting adverse clinical cardiovascular events, particularly in patients with anterior wall STEMI.
In conclusion, LAD was found to be independently associated with MACE occurrence following primary percutaneous coronary intervention during a 2.5-year follow-up period in patients with anterior wall STEMI, even in patients with normal LVEDD. This association was not found in patients with non-anterior wall STEMI. The present study highlights the importance of left atrial size in patients with STEMI, regardless of LVEDD, particularly in patients with anterior wall STEMI, for the prediction of adverse cardiovascular events.
Acknowledgements
The authors acknowledge all individuals who contributed to this research.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (No. XJTU1AF-CRF-2018-008).
ORCID iDs
Jianqing She https://orcid.org/0000-0001-7030-2889
Jun Lyu https://orcid.org/0000-0002-2237-8771
Hua Qiang https://orcid.org/0000-0001-6567-9681
References
- 1.Kapur NK, Alkhouli MA, DeMartini TJ, et al. Unloading the left ventricle before reperfusion in patients with anterior ST-segment-elevation myocardial infarction: a pilot study using the Impella CP. Circulation 2019; 139: 337–346. [DOI] [PubMed] [Google Scholar]
- 2.Kanjanahattakij N, Rattanawong P, Riangwiwat T, et al. Fragmented QRS and mortality in patients undergoing percutaneous intervention for ST-elevation myocardial infarction: systematic review and meta-analysis. Ann Noninvasive Electrocardiol 2018; 23: e12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu HW, Han YL, Jin QM, et al. One-year outcomes in patients with ST-segment elevation myocardial infarction caused by unprotected left main coronary artery occlusion treated by primary percutaneous coronary intervention. Chin Med J (Engl) 2018; 131: 1412–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zandecki L, Sadowski M, Janion M, et al. Survival benefit from recent changes in management of men and women with ST-elevation myocardial infarction treated with percutaneous coronary interventions. Cardiol J 2019; 26: 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosuge M, Kimura K. Clinical implications of electrocardiograms for patients with anterior wall ST-segment elevation acute myocardial infarction in the interventional era. Circ J 2012; 76: 32–40. [DOI] [PubMed] [Google Scholar]
- 6.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014; 63: 493–505. [DOI] [PubMed] [Google Scholar]
- 7.Saeed S, Gerdts E. Incremental prognostic value of left atrial function indices in the prediction of incident atrial fibrillation in patients with ST-elevation myocardial infarction. Int J Cardiol 2018; 263: 7–8. [DOI] [PubMed] [Google Scholar]
- 8.Lonborg JT, Engstrom T, Moller JE, et al. Left atrial volume and function in patients following ST elevation myocardial infarction and the association with clinical outcome: a cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging 2013; 14: 118–127. [DOI] [PubMed] [Google Scholar]
- 9.Sardana M, Lessard D, Tsao CW, et al. Association of left atrial function index with atrial fibrillation and cardiovascular disease: the Framingham offspring study. J Am Heart Assoc 2018; 7: pii: e008435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modin D, Olsen FJ, Pedersen S, et al. Measures of left atrial function predict incident atrial fibrillation in STEMI patients treated with primary percutaneous coronary intervention. Int J Cardiol 2018; 263: 1–6. [DOI] [PubMed] [Google Scholar]
- 11.Alosco ML, Gunstad J, Jerskey BA, et al. Left atrial size is independently associated with cognitive function. Int J Neurosci 2013; 123: 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anile M, Telha V, Diso D, et al. Left atrial size predicts the onset of atrial fibrillation after major pulmonary resections. Eur J Cardiothorac Surg 2012; 41: 1094–1097; discussion 1097. [DOI] [PubMed] [Google Scholar]
- 13.Kizer JR, Bella JN, Palmieri V, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS). Am Heart J 2006; 151: 412–418. [DOI] [PubMed] [Google Scholar]
- 14.Gerdts E, Wachtell K, Omvik P, et al. Left atrial size and risk of major cardiovascular events during antihypertensive treatment: losartan intervention for endpoint reduction in hypertension trial. Hypertension 2007; 49: 311–316. [DOI] [PubMed] [Google Scholar]
- 15.Piotrowski G, Banach M, Gerdts E, et al. Left atrial size in hypertension and stroke. J Hypertens 2011; 29: 1988–1993. [DOI] [PubMed] [Google Scholar]
- 16.Su G, Cao H, Xu S, et al. Left atrial enlargement in the early stage of hypertensive heart disease: a common but ignored condition. J Clin Hypertens (Greenwich) 2014; 16: 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjamin EJ, D’Agostino RB, Belanger AJ, et al. Left atrial size and the risk of stroke and death. The Framingham heart study. Circulation 1995; 92: 835–841. [DOI] [PubMed] [Google Scholar]
- 18.Spinelli L, Stabile E, Giugliano G, et al. Intramyocardial dissecting hematoma in anterior wall ST elevation myocardial infarction: impact on left ventricular remodeling and prognosis. Int J Cardiovasc Imaging 2018; 34: 201–210. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Zheng T, Wu H, et al. Predictive value of apelin-12 in patients with ST-elevation myocardial infarction with different renal function: a prospective observational study. BMJ Open 2017; 7: e018595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Zheng T, Wu H, et al. Data from: predictive value of apelin-12 in ST-elevation myocardial infarction patients with different renal function: a prospective observational study, Dryad, Dataset, 10.5061/dryad.pf56m (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry WL, DeMaria A, Gramiak R, et al. Report of the American Society of Echocardiography Committee on nomenclature and standards in two-dimensional echocardiography. Circulation 1980; 62: 212–217. [DOI] [PubMed] [Google Scholar]
- 22.Di Tullio MR, Sacco RL, Sciacca RR, et al. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke 1999; 30: 2019–2024. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi Y, Ohtani M, Sawa Y, et al. Left atrial diameter is a simple indicator of a deficiency in atrial natriuretic peptide secretion in patients with mitral stenosis: efficacy of postoperative supplementation with synthetic human alpha-atrial natriuretic peptide. J Cardiovasc Pharmacol 2004; 44: 709–717. [DOI] [PubMed] [Google Scholar]
- 24.Tsang TS, Abhayaratna WP, Barnes ME, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol 2006; 47: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 25.Seko Y, Kato T, Haruna T, et al. Association between atrial fibrillation, atrial enlargement, and left ventricular geometric remodeling. Sci Rep 2018; 8: 6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goette A, Kalman JM, Aguinaga L, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016; 18: 1455–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batra MK, Khan A, Farooq F, et al. Assessment of electrocardiographic criteria of left atrial enlargement. Asian Cardiovasc Thorac Ann 2018; 26: 273–276. [DOI] [PubMed] [Google Scholar]
- 28.Kaneshiro T, Yoshida K, Sekiguchi Y, et al. Crucial role of pulmonary vein firing as an initiator of typical atrial flutter: evidence of a close relationship between atrial fibrillation and typical atrial flutter. J Arrhythm 2017; 33: 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldmann KJ, Goldstein JA, Marinescu V, et al. Disparate impact of ischemic injury on regional wall dysfunction in acute anterior vs inferior myocardial infarction. Cardiovasc Revasc Med 2019; 20: 965–972. [DOI] [PubMed] [Google Scholar]
- 30.Sabia PJ, Powers ER, Jayaweera AR, et al. Functional significance of collateral blood flow in patients with recent acute myocardial infarction. A study using myocardial contrast echocardiography. Circulation 1992; 85: 2080–2089. [DOI] [PubMed] [Google Scholar]
- 31.Bogaert J, Bosmans H, Maes A, et al. Remote myocardial dysfunction after acute anterior myocardial infarction: impact of left ventricular shape on regional function - a magnetic resonance myocardial tagging study. J Am Coll Cardiol 2000; 35: 1525–1534. [DOI] [PubMed] [Google Scholar]
- 32.Beinart R, Boyko V, Schwammenthal E, et al. Long-term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol 2004; 44: 327–334. [DOI] [PubMed] [Google Scholar]