Abstract
Background
Fluid resuscitation has become the cornerstone of early septic shock management, but the optimal fluid rate is still not well studied. The goal of this investigation is to examine the relationship between fluid resuscitation rate and septic shock resolution.
Method
We retrospectively studied adult (≥ 18 years) patients with septic shock, defined based on sepsis III definition, from January 1, 2006, through May 31, 2018, in the medical intensive care unit (MICU) of Mayo Clinic Rochester. The fluid resuscitation time was defined as the time required to infuse the initial fluid bolus of 30 ml/kg, based on the recommendations of the 2016 surviving sepsis campaign. The cohort was divided into four groups based on the average fluid rate (group 1 ≥ 0.5, group 2 0.25–0.49, group 3 0.17–0.24, and group 4 < 0.17 ml/kg/min). The primary outcome was the time to shock reversal. Multivariable regression analyses were conducted to account for potential confounders.
Result
A total of 1052 patients met eligibility criteria and were included in the analysis. The time-to-shock reversal was significantly different among the groups (P < .001). Patients in group 1 who received fluid resuscitation at a faster rate had a shorter time to shock reversal (HR = 0.78; 95% CI 0.66–0.91; P = .01) when compared with group 4 with a median (IQR) time-to-shock reversal of 1.7 (1.5, 2.0) vs. 2.8 (2.6, 3.3) days, respectively. Using 0.25 ml/kg/min as cutoff, the higher fluid infusion rate was associated with a shorter time to shock reversal (HR = 1.22; 95% CI 1.06–1.41; P = .004) and with decreased odds of 28-day mortality (HR = 0.71; 95% CI 0.60–0.85; P < .001).
Conclusion
In septic shock patients, initial fluid resuscitation rate of 0.25–0.50 ml/kg/min (i.e., completion of the initial 30 ml/kg IV fluid resuscitation within the first 2 h), may be associated with early shock reversal and lower 28-day mortality compared with slower rates of infusion.
Keywords: Fluid resuscitation rate, Septic shock, Shock reversal, Vasopressor, Lactate clearance
The potential impact of this research
In this study, we assess the impact of the initial fluid replacement rate on the outcome of patients with septic shock managed based on Surviving Sepsis Campaign (SSC) guidelines. We found among septic shock patients the minimum initial fluid resuscitation rate of 0.25–0.50 ml/kg/min (i.e., completion of the initial 30 ml/kg IV fluid resuscitation within the first 2 h) is associated with a shorter time to shock reversal and improved patient outcome.
Introduction
Septic shock refers to sepsis with cardiovascular dysfunction. It is prevalent and is associated with a high rate of mortality [1, 2]. It is characterized by systemic vasodilation and increased vascular permeability [2–4]. These changes result in impaired microcirculatory blood flow and reduced tissue perfusion [5]. The fluid resuscitation for septic shock can restore perfusion before the onset of irreversible tissue damage [4] and prevent cardiovascular collapse and death [6, 7], and, hence, lower mortality [8]. Therefore, appropriate fluid resuscitation within the first 3 h of shock state is strongly recommended by the Surviving Sepsis Campaign (SSC) guidelines [9] as the cornerstone of septic shock treatment [10].
The challenge remains to identify the optimal fluid resuscitation strategy. While the standard of practice is the use of boluses of intravascular (IV) fluid for resuscitation [6, 9], a few trials have shown increased mortality with fluid resuscitation [11, 12]. Also, three recent multi-center randomized controlled trials [13–15] and a follow-up meta-analysis [16] showed that early goal-directed therapy (EGDT) bundles in comparison with usual care were not associated with improved outcomes in septic shock. Additionally, fluid boluses could lead to a positive fluid balance and excess fluid in the interstitial space [17, 18], resulting in tissue edema, decreased oxygen delivery, and increased mortality [19–21].
These controversies surrounding the optimal strategies of initial bolus-fluid administration during septic shock resuscitation make searching for the optimal dose, type, and rate of fluid resuscitation in septic shock a research priority [22]. The current guidelines recommend at least 30 ml/kg of intravenous crystalloid fluid to be given within the first 3 h of resuscitation [9, 23], but the influence may be different based on the time to attainment of this target in fluid resuscitation. So the goal of this study is to examine the relationship between initial fluid resuscitation rate and septic shock resolution in septic shock patients.
Methods
Participants
This is a retrospective cohort study of adult (≥ 18 years of age) medical intensive care unit (MICU) patients in Mayo Clinic, Rochester, MN, from January 1, 2006, through May 31, 2018, who had a diagnosis of septic shock and underwent resuscitation with IV fluids. Using hospital electronic health records (EHR), we screened MICU patients for eligibility. We identified septic shock patients who received fluid resuscitation > 30 ml/kg within the first 24 h and excluded patients with other types of shock [hypovolemic shock, cardiogenic shock, obstructive shock based on the International Classification of Diseases-10 (ICD-10) code of discharge diagnosis], those without Minnesota research authorization, vulnerable adults, prisoners, individuals with known pregnancy at the time of index admission, and patients who stayed in the MICU for < 48 h. The study was reviewed and approved by the Institutional Review Board (#18-008349) at Mayo Clinic, Rochester. Informed consent was waived for patients with Minnesota research authorization due to the minimal risk nature of the study.
Definitions
Sepsis was defined as an increase in the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score of 2 points or more, which caused by presumed or confirmed infection (Sepsis-3) [2]. To minimize the effect of temporal changes in the care of patients with sepsis during the study period and to confirm the accuracy of the included patients, the septic shock cases had to meet all three following criteria (1) diagnosis of septic shock based on ICD-10 code of discharge diagnosis, (2) criteria of sepsis described by Sepsis-3, and (3) mean arterial pressure (MAP) < 65 mmHg with vasopressor use and serum lactate level > 2 mmol/l along with an antibiotic prescription.
To minimize the effect of pre-hospital fluid resuscitation, we only included patients whose first recorded mean arterial pressure of < 65 mmHg occurred in MICU, and there was no record of vasopressor utilization prior to MICU admission. Time zero (T0) was defined as the first time that MAP was < 65 mmHg or serum lactate was > 2 mmol/l. Shock reversal time (Tr) was defined as the time in which MAP was > 65 mmHg without vasopressors and lactate < 2 mmol/l. Time to shock reversal was calculated as the duration between Tr and T0.
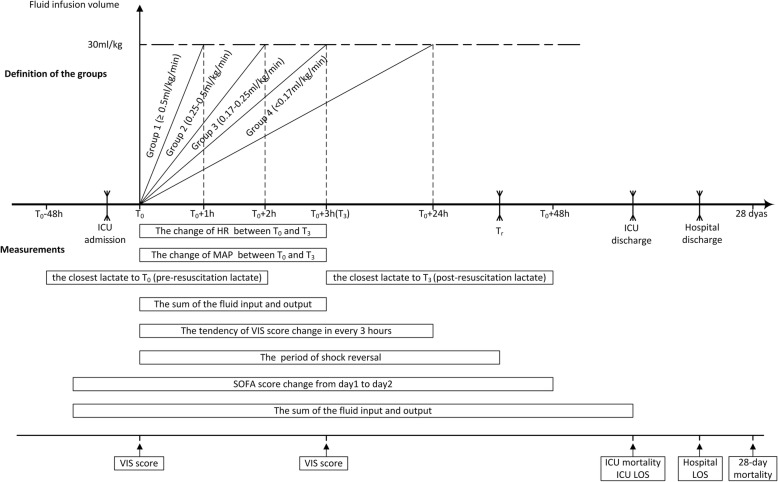
The initial fluid resuscitation rate (ml/kg/min) was calculated as the volume of 30 ml/kg of the actual body weight on admission divided by the time (min) to complete. Although the literature in support of the use of 30 ml/kg of crystalloid for initial volume resuscitation among septic shock patients is scarce, it is considered as one of the major recommendations by SSC2016. Therefore, we chose 30 ml/kg of crystalloid as a cutoff for the inclusion of patients in our study. In a recent study, investigators demonstrated that failure to deliver 30 ml/kg within 3 h of diagnosis of sepsis was associated with increased odds of in-hospital mortality, irrespective of other comorbidities [24]. Patients were categorized into four groups based on the resuscitation time: ≤ 1 h, 1.1–2 h, 2.1–3 h, and > 3 h for groups 1 to 4, respectively. The corresponding fluid rate for the groups described above were ≥ 0.5, 0.25–0.49, 0.17–0.24, and < 0.17 ml/kg/min, respectively (Fig. 1).
Fig. 1.
Schematic representation of the study protocol. Time zero (T0) was the starting point of septic shock fluid resuscitation and defined as the first time that MAP < 65 mmHg or serum lactate > 2 mmol/l during the ICU stay. According to the different range of initial fluid resuscitation rate (equal to the slope in the graph), the cohort was divided into four groups: group 1 (≥ 0.5 ml/kg/min), group 2 (0.25–0.5 ml/kg/min), group 3 (0.17–0.25 ml/kg/min), and group 4 (< 0.17 ml/kg/min). The “ ” on the timeline marked ICU admission, shock reversal time (Tr), and ICU and hospital discharge; shock reversal time (Tr), ICU discharge, and hospital discharge must be after T0 but had no fixed time relationship with T0. The main variables and measurements of the study are shown in the bottom half of the figure. The period of shock reversal was defined as the duration between Tr and T0 (abbreviation: ICU = intensive care unit; T0 = time zero; T3 = 3 h after T0; Tr = shock reversal time; VIS = Vasoactive-Inotropic Score; LOS = length of stay)
” on the timeline marked ICU admission, shock reversal time (Tr), and ICU and hospital discharge; shock reversal time (Tr), ICU discharge, and hospital discharge must be after T0 but had no fixed time relationship with T0. The main variables and measurements of the study are shown in the bottom half of the figure. The period of shock reversal was defined as the duration between Tr and T0 (abbreviation: ICU = intensive care unit; T0 = time zero; T3 = 3 h after T0; Tr = shock reversal time; VIS = Vasoactive-Inotropic Score; LOS = length of stay)
Pre-resuscitation lactate was defined as the lactate level closest (from 48 h before T0 to 2 h after T0) to T0, post-resuscitation lactate was defined as the lactate level closest to T3 (3 h after T0), and lactate clearance was determined by its decline between pre- and post-resuscitation lactate levels. The doses of the vasopressors were described by Vasoactive-Inotropic Score (VIS; [VIS = dopamine dose (mcg kg−1 min−1) + dobutamine dose (mcg kg−1 min−1) + 100 × epinephrine dose (mcg kg−1 min−1) + 10,000 × vasopressin dose (units kg−1 min−1) + 100 × norepinephrine dose (mcg kg−1 min−1) + 100 × phenylephrine dose (mcg kg−1 min−1)]) [25]. Fluid balance was defined as the difference of the fluid intake and output and was adjusted based on hospital admission weight. Acute Physiology And Chronic Health Evaluation (APACHE) III and SOFA scores were automatically calculated. Charlson comorbidity index (CCI) was determined at hospital admission.
Variables and outcomes
Baseline variables including patient demographics, hospital admission weight, hemodynamic variables, sites of infection, APACHE III and SOFA scores, and CCI were collected from the Multidisciplinary Epidemiology and Translational Research in Intensive Care (METRIC) DataMart [26]. The primary outcome was the time to shock reversal. Secondary outcomes included lactate clearance, weight-adjusted fluid balance in the first 3 h of resuscitation and throughout MICU stay, weight-adjusted fluid infusion between T3 and MICU discharge, timing of vasopressor initiation, temporal trends of VIS in the first 24 h calculated every 3 h, MAP and heart rate changes within the first 3 h of resuscitation, need and length of mechanical ventilation, SOFA day 1 to day 2 score changes, time to alive discharge from MICU and hospital, and finally MICU, hospital, and 28-day mortality (Fig. 1).
Statistical analysis
We summarized the data using frequencies and percentages for categorical variables and medians and interquartile ranges for continuous variables. We also compared data distributions across fluid resuscitation rate groups using chi-square and Kruskal-Wallis tests for categorical and continuous data, respectively.
Associations between fluid resuscitation rate and outcomes were analyzed using the univariable and multivariable models to adjust for age, sex, race, weight, CCI, APACHE III, and SOFA scores. We used logistic regression for binary outcomes (i.e., hospital and 28-day mortality) and linear regression for continuous outcomes (i.e., lactate clearance, fluid balance). The time to shock reversal and alive discharge from MICU, hospital, and 28 days were analyzed using Cox proportional hazards regression models. Associations between fluid resuscitation rate and VIS at different time points were analyzed using a multivariable generalized estimated equation model to adjust for the same variables listed previously. The median and interquartile range of VIS was plotted at hours 0, 6, 12, and 18 by fluid resuscitation completion time. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). A 2-sided P value < 0.05 was determined to be significant.
Result
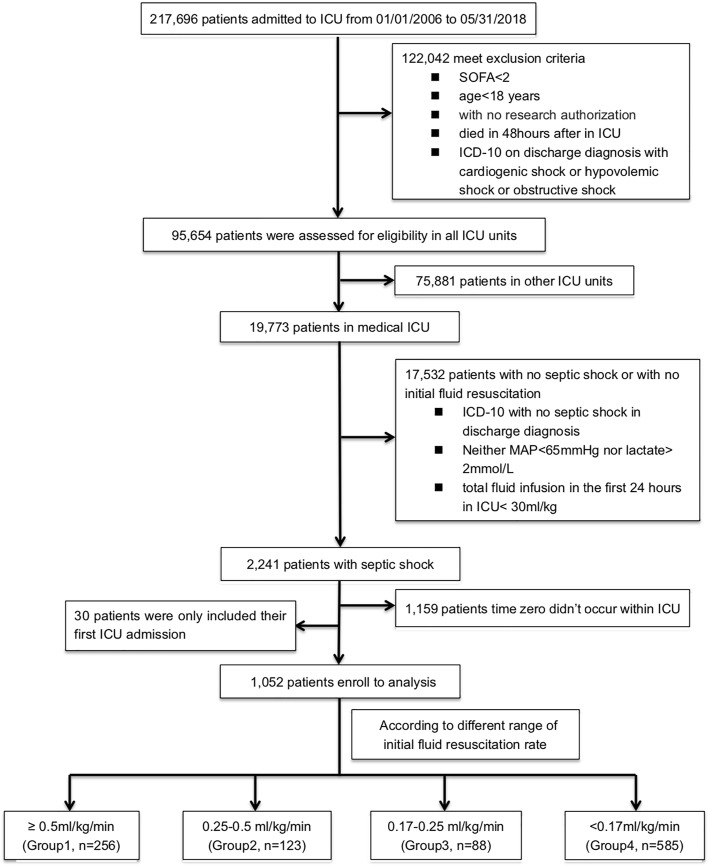
We screened 217,696 patients who were admitted to ICU from January 01, 2006, to May 31, 2018 (Fig. 2), and 1052 individuals who met all eligibility criteria entered the final analyses. Of those, 256 (24.3%) were in group 1, 123 (11.7%) in group 2, 88 (8.4%) in group 3, and 585 (55.6%) in group 4. Baseline characteristics for each category are presented in Table 1. Supplementary Figures 1A, B, and C show the average fluid intake, output, and balance, respectively, among all participants during the first 7 days of ICU admission. The four groups were similar with respect to demographic characteristics, comorbid conditions, and severity of illness. Patients with slower fluid resuscitation rates were heavier at baseline.
Fig. 2.
The CONSORT flow diagram of patient enrollment (abbreviation: ICU = intensive care unit; SOFA = Sequential [Sepsis-related] Organ Failure Assessment; ICD = International Classification of Diseases; MAP = mean arterial pressure)
Table 1.
Clinical demographics and baseline characteristics by initial fluid resuscitation rate (ml/kg/min)
| Variables | Fluid replacement rate; ml/kg/min | P value | |||
|---|---|---|---|---|---|
| ≥ 0. 5, N = 256 | 0.25–0.49, N = 123 | 0.17–0.24, N = 88 | < 0.17, N = 585 | ||
| Age; year, median (IQR) | 66 (55, 78) | 68 (59, 80) | 70 (53, 79) | 67 (56, 77) | .5† |
| Male sex; N (%) | 126 (49%) | 65 (53%) | 40 (46%) | 296 (51%) | .7‡ |
| White race; N (%) | 242 (95%) | 114 (93%) | 79 (90%) | 526 (90%) | .2‡ |
| Hospital admission weight; kg, median (IQR) | 76 (63, 90) | 81 (64, 100) | 80 (67, 103) | 84 (69, 99) | < .001† |
| Charlson comorbidity index; median (IQR) | 5 (3, 8) | 6 (4, 8) | 6 (3, 8) | 6 (4, 8) | .9† |
| APACHE III score (T0); median (IQR) | 82 (64, 104) | 83 (69, 106) | 86.5 (73, 107) | 86 (70, 103) | .3† |
| SOFA score (T0); median (IQR) | 8 (6, 11) | 9 (7, 12) | 9.0 (6.5, 11.0) | 9 (6, 12) | .4† |
| Infection source | |||||
| Blood | 73 (29%) | 32 (26%) | 25 (28%) | 155 (26%) | .9 |
| Respiratory | 95 (37%) | 38 (31%) | 37 (43%) | 274 (47%) | .002 |
| Abdominal/GI | 44 (17%) | 13 (11%) | 10 (11%) | 78 (13%) | .2 |
| Urinary | 55 (21%) | 28 (23%) | 32 (37%) | 135 (23%) | .02 |
| Soft tissue and bone | 24 (9%) | 11 (9%) | 13 (15%) | 41 (7%) | .08 |
| Other | 23 (9%) | 13 (11%) | 12 (14%) | 54 (9%) | .5 |
| Heart rate (T0), bpm; median (IQR) | 101 (88, 116) | 100 (83, 113) | 105 (88, 117) | 98 (83, 111) | .007 |
| Mean arterial pressure (T0), mmHg; median (IQR) | 64 (57, 73) | 60 (54, 68) | 65 (57, 75) | 66 (59, 76) | < .001 |
| VIS (T0); median (IQR) | 1.6 (0.5, 10.1) | 1.5 (0.6, 12.8) | 1.7 (0.7, 7.2) | 1.7 (0.6, 10.6) | .9 |
Numbers indicate N (%) unless otherwise noted
†Kruskal-Wallis
‡Chi-square
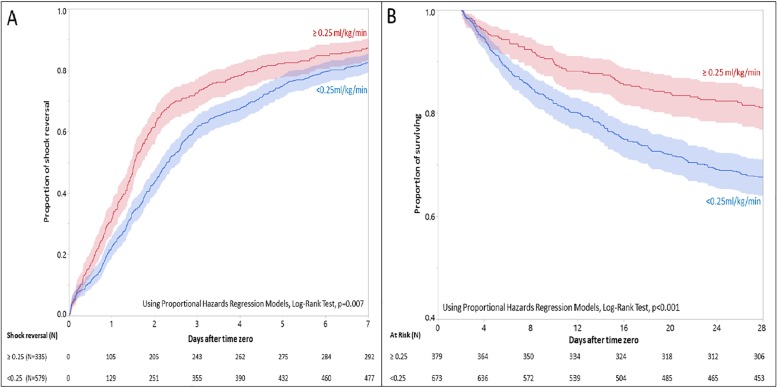
Among the groups, 91, 84, 83, and 87% achieved shock reversal in groups 1 to 4, respectively (P = .2). However, the time to shock reversal was significantly different (P < .001) among the groups. In multivariable analyses after adjustment for a priori independent variables, patients in group 1 achieved shock reversal faster compared to patients in group 4 (HR = 0.78; 95% CI 0.66–0.91) with a median (IQR) time to shock reversal of 1.7 (1.5, 2.0) vs. 2.8 (2.6, 3.3) days, respectively. When we used the year of admission in the multivariable models, it was not a significant predictor of outcomes (results are not shown). Time to shock reversal was not different when we compared groups 2 and 3 with group 1 (Table 2). Using 0.25 ml/kg/min as threshold, more patients with a higher fluid rate (≥ 0.25 ml/kg/min) achieved shock reversal (HR = 1.22; 95% CI 1.06–1.41; P = .004) with a significant shorter median (IQR) time to shock reversal [1.5 (1.4, 1.7) vs. 2.3 (2.1, 2.6) days] compared to patients with a lower fluid rate (< 0.25 ml/kg/min) (Fig. 3a).
Table 2.
Comparison of outcomes by four different initial fluid resuscitation rate (ml/kg/min)
| Variables | Fluid replacement rate; ml/kg/min | ||||
|---|---|---|---|---|---|
| ≥ 0.5, N = 256 | 0.25–0.49, N = 123 | 0.17–0.24, N = 88 | < 0.17, N = 585 | P value | |
| Time to shock reversal; days | < .001‡ | ||||
| N | 232 | 103 | 73 | 506 | |
| Median (Q1, Q3) | 1.6 (0.9, 3.3) | 1.4 (0.6, 3.2) | 2.0 (0.9, 4.6) | 2.4 (1.2, 5.1) | |
| Median survival | 1.72 (1.52, 2.01) | 1.87 (1.32, 2.62) | 2.58 (2.02, 4.40) | 2.80 (2.57, 3.26) | .004§ |
| Adjusted hazard ratioa | 1.00 (ref) | 0.96 (0.76, 1.21) | 0.84 (0.65, 1.10) | 0.78 (0.66, 0.91) ** | .01¶ |
| Lactate clearance; mg/dl | < .001‡ | ||||
| N | 207 | 97 | 62 | 400 | |
| Median (Q1, Q3) | 0.5 (− 0.1, 1.3) | 0.4 (− 0.05, 1.2) | 0.6 (− 0.04, 1.2) | 0.2 (− 0.4, 0.8) | |
| Adjusted mean estimate c | 0.00 (ref) | 0.05 (− 0.27, 0.38) | 0.13 (− 0.25, 0.52) | − 0.45 (− 0.68, − 0.23)*** | < .001¶ |
| MAP change in the first 3 h; mmHg | < .001 ‡ | ||||
| N | 254 | 120 | 79 | 508 | |
| Median (Q1, Q3) | 4.3 (− 3.0, 11.5) | 4.0 (− 4.5, 11.5) | − 0.5 (− 10.5, 7.0) | 0.5 (− 5.5, 7.0) | |
| Adjusted mean estimatec | 0.00 (ref) | − 1.14 (− 4.25, 1.96) | − 3.55 (− 7.17, 0.07) | − 2.93 (− 5.10, − 0.75)** | .04¶ |
| HR change in the first 3 h; bpm | .2‡ | ||||
| N | 255 | 120 | 82 | 545 | |
| Median (Q1, Q3) | − 2.0 (− 7.5, 2.5) | − 2.3 (− 8.0, 4.3) | − 2.5 (− 9.5, 0.5) | − 1.5 (− 7.5, 3.5) | |
| Adjusted mean estimatec | 0.00 (ref) | 0.50 (− 2.06, 3.07) | − 1.41 (− 4.37, 1.54) | 1.63 (− 0.15, 3.41) | .08¶ |
| Fluid balance at T3; ml/kg | 59 (45, 79) | 44 (35, 60) | 33 (31, 41) | 7 (1, 15) | < .001‡ |
| Adjusted mean estimatec | 0.00 (ref) | 14.61 (10.63, 18.59)*** | 28.73 (24.23, 33.22)*** | 57.08 (54.34, 59.82)*** | < .001¶ |
| MICU fluid balance; ml/kg | 76 (48, 136) | 71 (40, 125) | 66 (24, 146) | 50 (16, 110) | < .001‡ |
| Adjusted mean estimatec | 0.00 (ref) | − 10 (− 34, 14) | − 6 (− 33, 21) | 13 (− 4, 29) | .1¶ |
| Fluid infusion between T3 and MICU discharge; ml/kg | 107 (50, 264) | 124 (49, 223) | 158 (62, 381) | 168 (86, 345) | < .001‡ |
| Adjusted mean estimatec | 0.00 (ref) | − 54 (− 102, − 5)* | 37 (− 18, 91) | 59 (27, 90)*** | < .001¶ |
| Duration between first vasopressor using and T0; hours | < .001‡ | ||||
| N | 233 | 102 | 75 | 502 | |
| Median (Q1, Q3) | 2.0 (0.5, 4.7) | 2.3 (1.3, 3.9) | 3.7 (1.4, 6.7) | 7.0 (2.2, 17.7) | |
| Adjusted mean estimatec | 0.00 (ref) | − 3.8 (− 12.5, 5.0) | − 2.0 (− 11.7, 7.8) | 10.3 (4.6, 15.9)*** | < .001¶ |
| SOFA change (day 2–day 1) | < .001‡ | ||||
| N | 240 | 114 | 80 | 561 | |
| Median (Q1, Q3) | − 3 (− 5, − 1) | − 2 (− 5, − 1) | − 2 (− 3.5, − 0.5) | − 2 (− 4, 0) | |
| Adjusted mean estimatec | 0.00 (ref) | − 0.09 (− 0.71, 0.53) | 0.85 (0.15, 1.55)* | 0.94 (0.52, 1.36)*** | < .001¶ |
| Mechanical ventilation; N (%) | 134 (52%) | 68 (55%) | 55 (63%) | 383 (66%) | .002† |
| Adjusted odds ratiob | 1.00 (ref) | 1.04 (0.63, 1.75) | 1.56 (0.87, 2.83) | 1.91 (1.34, 2.73)*** | .001¶ |
| Mechanical ventilation duration; days | .002‡ | ||||
| N | 134 | 68 | 55 | 383 | |
| Median (Q1, Q3) | 3 (1, 7) | 2 (1, 6) | 5 (2, 9) | 4 (2, 8) | |
| Adjusted mean estimatec | 0.00 (ref) | − 14.95 (− 66.76, 36.87) | 43.67 (− 11.93, 99.27) | 45.07 (9.60, 80.54)* | .01¶ |
| Discharge alive from MICU | 238 (93%) | 112 (91%) | 81 (92%) | 481 (82%) | < .001† |
| Median survival; days | 2.89 (2.64, 3.19) | 3.09 (2.42, 3.86) | 4.06 (3.12, 5.99) | 4.90 (4.51, 5.67) | < .001§ |
| Adjusted hazard ratioa | 1.00 (ref) | 0.95 (0.75, 1.19) | 0.74 (0.57, 0.95)* | 0.58 (0.49, 0.68)*** | < .001¶ |
| Discharge alive from hospital | 225 (88%) | 93 (76%) | 68 (77%) | 415 (71%) | < .001† |
| Median survival; days | 10.09 (8.51, 11.80) | 12.51 (9.77, 15.03) | 16.81 (13.66, 21.01) | 17.69 (15.45, 19.96) | < .001§ |
| Adjusted hazard ratioa | 1.00 (ref) | 0.84 (0.66, 1.08) | 0.58 (0.44, 0.76)*** | 0.53 (0.45, 0.63)*** | < .001¶ |
| MICU mortality; N (%) | 18 (7%) | 11 (9%) | 7 (8%) | 104 (18%) | < .001† |
| Adjusted odds ratiob | 1.00 (ref) | 1.34 (0.59, 3.03) | 1.12 (0.44, 2.87) | 3.40 (1.95, 5.93)*** | < .001¶ |
| Hospital mortality; N (%) | 31 (12%) | 30 (24%) | 20 (23%) | 170 (29%) | < .001† |
| Adjusted odds ratiob | 1.00 (ref) | 2.53 (1.41, 4.52)** | 2.23 (1.17, 4.27)* | 3.51 (2.27, 5.44)*** | < .001¶ |
| 28-day mortality; N (%) | 41 (16%) | 32 (26%) | 21 (24%) | 199 (34%) | < .001† |
| Adjusted odds ratiob | 1.00 (ref) | 1.97 (1.12, 3.45)* | 1.86 (0.99, 3.51) | 3.19 (2.13, 4.78)*** | < .001¶ |
Numbers indicate N (%) and median (Q1, Q3) unless otherwise noted
Unadjusted models contain just fluid resuscitation rate adjusted models are additionally adjusted for the effects of age, gender, white race, weight, CCI, APACHE III, and SOFA
Compared to ≥ 0. 5 ml/kg/min group, *P value < 0.05, **P value < 0.01, ***P value < 0.001
†Chi-square
‡Kruskal-Wallis
§Log-rank test
¶Type 3 Wald test
aAnalyzed using proportional hazards regression models
bAnalyzed using logistic regression models
cAnalyzed using linear regression models
Fig. 3.
The cumulative proportion of shock reversal and survival analysis with different initial fluid resuscitation rates (≥ 0.25 ml/kg/min vs. < 0.25 ml/kg/min). The shade indicates the confidence interval. a The cumulative proportion of shock reversal. Using Cox-model (additionally adjusted for the effects of age, gender, white race, weight, CCI, APACHE III, and SOFA), patients with ≥ 0.25 ml/kg/min rate had higher proportion of shock reversal (HR = 1.22; 95% CI 1.06–1.41; P = .007), with shorter median time (IQR) to shock reversal [1.5 (1.4, 1.7) vs. 2.3 (2.1, 2.6) days] for patients with < 0.25 ml/kg/min rate. b Survival analysis. Using Cox-model (additionally adjusted for the effects of age, gender, white race, weight, CCI, APACHE III, and SOFA), patients with ≥ 0.25 ml/kg/min rate had higher proportion of surviving (HR = 0.71; 95% CI 0.60–0.85; P < .001) for patients with < 0.25 ml/kg/min rate
Secondary outcomes
The time of pre-resuscitation reported lactate was 0 (− 3.6, 1.1) h from T0, and the time of post-resuscitation reported lactate was 3.8 (1.5, 10.6) h after T3. The lactate clearance during initial fluid resuscitation was significantly different among groups (P < .001); group 4 had less lactate reduction compared to group 1 (0.2 vs. 0.5 mg/dl; P < .001). Group 4 also had minimal change in MAP at 3 h, compared to group 1, who had a median increase of 4.3 mmHg (P = .04). A lower initial fluid resuscitation rate was associated with a later vasopressor use after septic shock onset (P < .001). A higher initial fluid resuscitation rate was associated with a higher mean fluid balance at 3 h (P < .001), but not with the mean fluid balance for the rest of the MICU stay (P = .1). The volume of infused fluid from T3 to the MICU discharge was significantly higher in group 4 compared to groups 1 (168 vs. 107 ml/kg, P < .001) and 2 (168 vs. 124 ml/kg, P < .001). Relative to group 4, fewer patients in group 1 required mechanical ventilation (OR = 1.91; 95% CI = 1.34, 2.73; P = .001), and for the ones who needed mechanical ventilation, the duration was shorter (mean = 1.88; 95% CI = 0.40, 3.36 days; P = .01). Group 1 also had a more significant decline in SOFA score compared to groups 3 (− 3 vs. − 2; P < .05) and 4 (− 3 vs. − 2, P < .001) (Table 2).
The proportions of alive discharges from ICU and hospital (P < .001 for both) significantly differed among the groups. Similarly, MICU (P < .001) and hospital durations (P < .001) were different. Compared to group 4, group 1 had a shorter MICU (2.9 vs.4.9 days; P < .001) and hospital (10.1 vs. 17.7 days; P < .001) stay. The 28-day mortality was also significantly different among the groups (P < .001). Compared to group 4, group 1 had lower 28-day mortality (16.0% vs.34.0%; P < .001) (Table 2). Using 0.25 ml/kg/min as threshold for intravascular fluid replacement rate, patients with a higher fluid rate (≥ 0.25 ml/kg/min) were more likely to survive at 28 days (HR = 0.71; 95% CI 0.60–0.85; P < .001) compared to ones with a lower fluid rate (< 0.25 ml/kg/min) (Fig. 3b).
Table 3 and Fig. 4 show associations between fluid resuscitation rate and VIS at various time points. Fluid rate of < 0.17 ml/kg/min and increased weight were associated with lower VIS scores at all time points. Increased length of ICU stay, APACHE III, and SOFA scores were associated with increased VIS scores.
Table 3.
Associations between fluid time and VIS by hour using univariate and multivariable GEE linear regression models
| Variables | Univariate analysis† | Multivariable analysis‡ | ||
|---|---|---|---|---|
| Mean estimate (95% CI) | P value | Mean estimate (95% CI) | P value | |
| Fluid replacement rate; ml/kg/min | < .001 | < .001 | ||
| ≥ 0.5 | 0.00 (ref) | 0.00 (ref) | ||
| 0.25–0.49 | 0.18 (− 1.89, 2.24) | 0.11 (− 1.77, 2.00) | ||
| 0.17–0.24 | − 0.03 (− 2.34, 2.28) | − 0.15 (− 2.23, 1.92) | ||
| < 0.17 | − 2.62 (− 4.08, − 1.16) | − 2.41 (− 3.79, − 1.03) | ||
| VIS time (per 1 h) | 0.20 (0.15, 0.24) | < .001 | 0.18 (0.13, 0.22) | < .001 |
| Age (per year) | − 0.01 (− 0.05, 0.03) | .6 | − 0.03 (− 0.08, 0.02) | .2 |
| Male sex | − 0.07 (− 1.25, 1.11) | .9 | 0.33 (− 0.74, 1.41) | .5 |
| White race | − 0.73 (− 2.79, 1.34) | .5 | − 0.24 (− 2.18, 1.70) | .8 |
| Weight (per 10 kg) | − 0.5 (− 0.7, − 0.4) | < .001 | − 0.5 (− 0.7, − 0.3) | < .001 |
| Charlson comorbidity index | 0.02 (− 0.19, 0.23) | .9 | 0.04 (− 0.18, 0.27) | .7 |
| APACHE III score | 0.09 (0.07, 0.11) | < .001 | 0.05 (0.01, 0.08) | .007 |
| SOFA | 0.68 (0.52, 0.84) | < .001 | 0.41 (0.16, 0.66) | < .001 |
†Mean estimate and P value are adjusted only for the row covariate
‡Mean estimate and P value are adjusted for all covariates listed in the table
Fig. 4.
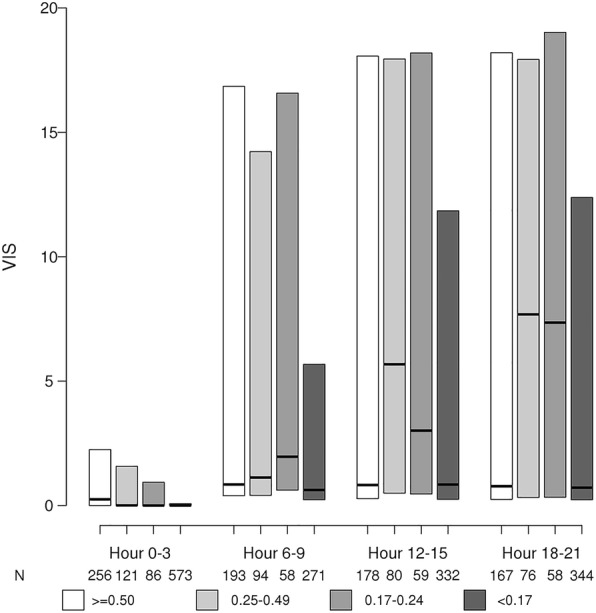
Association between fluid resuscitation rate (ml/kg/min) and VIS at variable time periods during the first 24 h of fluid resuscitation. Group < 0.17 ml/kg/min had significantly lower VIS at all time period. Compared to group < 0.17 ml/kg/min, *P value < 0.05, **P value < 0.01 (abbreviation: VIS = Vasoactive-Inotropic Score)
Discussion
In this retrospective analysis of MICU patients with septic shock who received fluid resuscitation, we demonstrated that a fluid resuscitation rate > 0.25 ml/kg/min was associated with a shorter time to shock reversal and lower 28-day mortality. Based on our observations, the optimal fluid rate was 0.25–0.5 ml/kg/min (equivalent to 30 ml/kg fluid administration within 2 h). Faster initial fluid resuscitation, in our study, was also associated with lower 28-day mortality. This finding is consistent with a recent prospective observational cohort study, which found that sepsis patients who received initial fluid resuscitation in > 2 h after diagnosis had increased mortality rates [27]. Vincent also suggested administration of 30 ml/kg of fluid over 3 h to be too slow for appropriate fluid resuscitation [28].
The goal of initial fluid resuscitation in septic shock is to restore intravascular volume, cardiac output, and oxygen delivery [29]. To restore intravascular volume, the rate of initial fluid infusion should be faster than the speed of fluid loss via leaky vascular endothelium. A faster initial fluid resuscitation rate could also decrease the early inflammation [30] and blood viscosity [31] to improve the microcirculation and tissue perfusion [4]. A higher fluid rate is also associated with a more significant increase in MAP and a greater reduction in lactate, a marker for tissue perfusion [32]. This finding is consistent with previous reports that showed improved microcirculation in sepsis following the early administration of fluids [33]. The improvement both in macrocirculation and microcirculation can lead to improved outcomes including less time to shock reversal, need and duration of mechanical ventilation, SOFA score, hospital, MICU stay, and 28-day mortality rates.
On the other hand, very fast fluid replacement may increase glycocalyx shedding and exacerbate vascular dysfunction. The endothelial glycocalyx layer is damaged during sepsis, which negatively impacts its barrier function [34–36]. Receiving 40–60 ml/kg IV fluid boluses in 1 h (up to 0.67–1 ml/kg/min) [11] among critically ill children led to a cardiovascular collapse in a large trial [19]. Bryne and colleagues found a rapid increase in vasopressor requirement and a significant increase in glycocalyx layer damage after initial fluid resuscitation in an ovine model of endotoxemia following fluid infusion rate of 0.67 ml/kg/min [37]. In our study, while we noted rapid fluid replacement is associated with improved outcomes of septic shock, we were not able to assess the impact of very high fluid rates (> 0.67 ml/kg/min).
We also identified an association between a lower initial fluid resuscitation rate and a lower VIS at various time points in the first 24 h of septic shock onset. This result was most likely due to later initiation of vasopressors and the impact of higher baseline weight in calculating VIS [38–40]. A higher VIS at 48 h after cardiovascular surgery was found to be associated with a longer ICU length of stay and longer ventilator days in pediatric sepsis patients [41]. In our study, group 4 with a lower fluid resuscitation rate and a lower VIS in the first 24 h had worse clinical outcomes. This discrepancy could be due to not assessing VIS beyond 24 h. Also, the relationship between VIS and patient outcomes has not been validated in sepsis.
In our study, a faster initial fluid resuscitation rate was associated with a larger positive fluid balance in the first 3 h of resuscitation, but the differences in fluid balance among the groups were not significant at MICU discharge. Treating patients with septic shock inevitably would result in initial positive fluid balance, which in the early phases of fluid resuscitation increases cardiac output in most patients [42]. In a study by Lee et al., septic shock patients who received a larger volume of fluid in the first 3 h were more likely to survive [43]. The differences in positive fluid balance among the groups resolved beyond the first 3 h because group 1 received fewer fluids after the initial resuscitation phase. Patients who receive less fluid in the first 6 h have significantly higher fluid balance in the next 7 to 72 h, in-hospital, and 28-day mortality [44]. Shen and colleagues also showed a positive fluid balance during the second, but not the first 24 h of septic shock was associated with increased mortality in sepsis [45]. Positive fluid balance during the initial resuscitation phase is associated with the improved outcome as long as the fluid balance is carefully monitored after the resuscitation targets are met.
Our study has several limitations. Due to its retrospective design, we are not able to imply any causal relationship [46]. Hence, prospective studies are required to verify our results. We included patients whose first recorded MAP of < 65 mmHg happened in MICU and had no record of vasopressor utilization prior to MICU admission. Despite this, we still could not completely eliminate the effect of potential fluid administration prior to the MICU admission. There is growing knowledge indicating that fluid resuscitation should be guided by fluid responsiveness [47]. Meanwhile, as one of the limitations of our study, we were not able to acquire fluid responsiveness data. As SSC guidelines indicate the use of 30 ml/kg to septic shock patients, we believe our results are still within recommended guidelines even though it is not based on fluid responsiveness assessment. In our institution, the administration of the second or third bolus of 30 ml/kg of fluids is only guided by the fluid responsiveness assessment. Lastly, the study period spanned 12 years, and changes in the clinical practice could have led to bias in our results.
Conclusion
In septic shock patients, the minimum fluid resuscitation rate of 0.25–0.50 ml/kg/min (i.e., completion of the initial 30 ml/kg IV fluid resuscitation within the first 2 h) is associated with a shorter time to shock reversal and improved patient clinical outcomes. Our findings would serve as hypothesis-generating information in order to design and conduct prospective trials for validation.
Supplementary information
Additional file 1 Supplementary Figure 1. Fluid assessment in the first seven days after time zero; A) fluid input, B) fluid output, C) fluid balance.
Acknowledgements
None
Authors’ contributions
BH, ZP, and KK designed this study and protocol development. MP and EP were responsible for the data collection. RF and BH were responsible for data analysis. BH and JC conducted the manuscript writing. KK, RF, YD, and ZP critically revised the manuscript. BH, JC, KK, and ZP provided final approval for this version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors read and approved the final manuscript.
Funding
Mayo Clinic Rochester CCRS (Critical Care IMP Research Subcommittee) small grant for statistical support.
Availability of data and materials
The data used for this research are available from the corresponding author on reasonable request and subject to Institutional Review Board guidelines.
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Review Board (#18-008349) at Mayo Clinic, Rochester.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhiyong Peng, Email: pengzy5@hotmail.com.
Kianoush Kashani, Email: Kashani.kianoush@mayo.edu.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13054-020-2819-5.
References
- 1.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 4.Rivers EP, Jaehne AK, Eichhorn-Wharry L, Brown S, Amponsah D. Fluid therapy in septic shock. Curr Opin Crit Care. 2010;16:297–308. doi: 10.1097/MCC.0b013e32833be8b3. [DOI] [PubMed] [Google Scholar]
- 5.Gupta RG, Hartigan SM, Kashiouris MG, Sessler CN, Bearman GM. Early goal-directed resuscitation of patients with septic shock: current evidence and future directions. Crit Care. 2015;19:286. doi: 10.1186/s13054-015-1011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46:997–1000. doi: 10.1097/CCM.0000000000003119. [DOI] [PubMed] [Google Scholar]
- 7.Rivers EP, Coba V, Visbal A, Whitmill M, Amponsah D. Management of sepsis: early resuscitation. Clin Chest Med. 2008;29:689–704. doi: 10.1016/j.ccm.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, Osborn T, Lemeshow S, Chiche JD, Artigas A, Dellinger RP. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med. 2015;43:3–12. doi: 10.1097/CCM.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Int Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 10.Loflin R, Winters ME. Fluid resuscitation in severe sepsis. Emerg Med Clin North Am. 2017;35:59–74. doi: 10.1016/j.emc.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, Evans JA, Tibenderana JK, Crawley J, Russell EC, Levin M, Babiker AG, Gibb DM, Group FT Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 12.Andrews B, Semler MW, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, Mabula C, Bwalya M, Bernard GR. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMA. 2017;318:1233–1240. doi: 10.1001/jama.2017.10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pro CI, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 15.Investigators A, Group ACT. Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 16.Investigators P, Rowan KM, Angus DC, Bailey M, Barnato AE, Bellomo R, Canter RR, Coats TJ, Delaney A, Gimbel E, Grieve RD, Harrison DA, Higgins AM, Howe B, Huang DT, Kellum JA, Mouncey PR, Music E, Peake SL, Pike F, Reade MC, Sadique MZ, Singer M, Yealy DM. Early, goal-directed therapy for septic shock - a patient-level meta-analysis. N Engl J Med. 2017;376:2223–2234. doi: 10.1056/NEJMoa1701380. [DOI] [PubMed] [Google Scholar]
- 17.Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med. 2017;43:625–632. doi: 10.1007/s00134-016-4675-y. [DOI] [PubMed] [Google Scholar]
- 18.Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015;19:251. doi: 10.1186/s13054-015-0970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, Opoka RO, Engoru C, Nyeko R, Mtove G, Reyburn H, Brent B, Nteziyaremye J, Mpoya A, Prevatt N, Dambisya CM, Semakula D, Ddungu A, Okuuny V, Wokulira R, Timbwa M, Otii B, Levin M, Crawley J, Babiker AG, Gibb DM. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;11:68. doi: 10.1186/1741-7015-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 21.Vaara ST, Korhonen AM, Kaukonen KM, Nisula S, Inkinen O, Hoppu S, Laurila JJ, Mildh L, Reinikainen M, Lund V, Parviainen I, Pettila V, Group FS Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit Care. 2012;16:R197. doi: 10.1186/cc11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coopersmith CM, De Backer D, Deutschman CS, Ferrer R, Lat I, Machado FR, Martin GS, Martin-Loeches I, Nunnally ME, Antonelli M, Evans LE, Hellman J, Jog S, Kesecioglu J, Levy MM, Rhodes A. Surviving sepsis campaign: research priorities for sepsis and septic shock. Intensive Care Med. 2018;44(9):1400–26. [DOI] [PMC free article] [PubMed]
- 23.Hilton AK, Bellomo R. A critique of fluid bolus resuscitation in severe sepsis. Crit Care. 2012;16:302. doi: 10.1186/cc11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuttab HI, Lykins JD, Hughes MD, Wroblewski K, Keast EP, Kukoyi O, Kopec JA, Hall S, Ward MA. Evaluation and predictors of fluid resuscitation in patients with severe sepsis and septic shock. Crit Care Med. 2019. [DOI] [PMC free article] [PubMed]
- 25.Yamazaki Y, Oba K, Matsui Y, Morimoto Y. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth. 2018;32(2):167–73. [DOI] [PubMed]
- 26.Herasevich V, Pickering BW, Dong Y, Peters SG, Gajic O. Informatics infrastructure for syndrome surveillance, decision support, reporting, and modeling of critical illness. Mayo Clin Proc. 2010;85:247–254. doi: 10.4065/mcp.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leisman DE, Doerfler ME, Schneider SM, Masick KD, D'Amore JA, D'Angelo JK. Predictors, prevalence, and outcomes of early crystalloid responsiveness among initially hypotensive patients with Sepsis and septic shock. Crit Care Med. 2018;46:189–198. doi: 10.1097/CCM.0000000000002834. [DOI] [PubMed] [Google Scholar]
- 28.Vincent J-L. Fluid management in the critically ill. Kidney Int. 2019;96(1):52-7. [DOI] [PubMed]
- 29.Semler MW, Rice TW. Sepsis resuscitation: fluid choice and dose. Clin Chest Med. 2016;37:241–250. doi: 10.1016/j.ccm.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorresteijn MJ, van Eijk LT, Netea MG, Smits P, van der Hoeven JG, Pickkers P. Iso-osmolar prehydration shifts the cytokine response towards a more anti-inflammatory balance in human endotoxemia. J Endotoxin Res. 2005;11:287–293. doi: 10.1177/09680519050110050501. [DOI] [PubMed] [Google Scholar]
- 31.Grocott MP, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100:1093–1106. doi: 10.1213/01.ANE.0000148691.33690.AC. [DOI] [PubMed] [Google Scholar]
- 32.Napoli AM, Seigel TA. The role of lactate clearance in the resuscitation bundle. Crit Care. 2011;15:199. doi: 10.1186/cc10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Buchele G, Simion D, Chierego ML, Silva TO. Fonseca A, Vincent JL, De Backer D. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36:949–955. doi: 10.1007/s00134-010-1843-3. [DOI] [PubMed] [Google Scholar]
- 34.Chappell D, Jacob M. Role of the glycocalyx in fluid management: small things matter. Best Pract Res Clin Anaesthesiol. 2014;28:227–234. doi: 10.1016/j.bpa.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Hao D, Wang T, Gao F, Sun T, Li Y, Lu F, Liu X, Hu Z, Lyu C, Wang X. Variation and clinical value of endothelial glycocalyx in the patients with septic shock. Zhonghua wei zhong bing ji jiu yi xue. 2016;28:699–703. doi: 10.3760/cma.j.issn.2095-4352.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Inagawa R, Okada H, Takemura G, Suzuki K, Takada C, Yano H, Ando Y, Usui T, Hotta Y, Miyazaki N, Tsujimoto A, Zaikokuji R, Matsumoto A, Kawaguchi T, Doi T, Yoshida T, Yoshida S, Kumada K, Ushikoshi H, Toyoda I, Ogura S. Ultrastructural alteration of pulmonary capillary endothelial glycocalyx during endotoxemia. Chest. 2018;154(2):317-25. [DOI] [PubMed]
- 37.Byrne L, Obonyo NG, Diab SD, Dunster KR, Passmore MR, Boon AC, Hoe LS, Pedersen S, Fauzi MH, Pimenta LP, Van Haren F, Anstey CM, Cullen L, Tung JP, Shekar K, Maitland K, Fraser JF. Unintended consequences: fluid resuscitation worsens shock in an ovine model of endotoxemia. Am J Respir Crit Care Med. 2018;198:1043–1054. doi: 10.1164/rccm.201801-0064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, Charpie JR, Hirsch JC. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11:234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 39.Barge-Caballero E, Segovia-Cubero J, Gonzalez-Vilchez F, Delgado-Jimenez J, Perez-Villa F, Almenar-Bonet L, Arizon-Del Prado JL, Lage-Galle E, De La Fuente-Galan L, Manito-Lorite N, Sanz-Julve M, Villa-Arranz A, Lambert Rodriguez JL, Brossa-Loidi V, Pascual-Figal D, Muniz-Garcia J, Crespo-Leiro M. Evaluation of the preoperative vasoactive-inotropic score as a predictor of postoperative outcomes in patients undergoing heart transplantation. Int J Cardiol. 2015;185:192–194. doi: 10.1016/j.ijcard.2015.03.098. [DOI] [PubMed] [Google Scholar]
- 40.Gaies MG, Jeffries HE, Niebler RA, Pasquali SK, Donohue JE, Yu S, Gall C, Rice TB, Thiagarajan RR. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med. 2014;15:529–537. doi: 10.1097/PCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIntosh AM, Tong S, Deakyne SJ, Davidson JA, Scott HF. Validation of the vasoactive-inotropic score in pediatric sepsis. Pediatr Crit Care Med. 2017;18:750–757. doi: 10.1097/PCC.0000000000001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malbrain M, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal PJ, Joannes-Boyau O, Teboul JL, Rice TW, Mythen M, Monnet X. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018;8:66. doi: 10.1186/s13613-018-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SJ, Ramar K, Park JG, Gajic O, Li G, Kashyap R. Increased fluid administration in the first three hours of sepsis resuscitation is associated with reduced mortality: a retrospective cohort study. Chest. 2014;146:908–915. doi: 10.1378/chest.13-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative G. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 45.Shen Y, Ru W, Huang X, Zhang W. Time-related association between fluid balance and mortality in sepsis patients: interaction between fluid balance and haemodynamics. Sci Rep. 2018;8:10390. doi: 10.1038/s41598-018-28781-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer E, Klapaukh R, Harris S, Singer M. Intelligently learning from data. Crit Care. 2019;23:136. doi: 10.1186/s13054-019-2424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marik PE. Fluid responsiveness and the six guiding principles of fluid resuscitation. Crit Care Med. 2016;44:1920–1922. doi: 10.1097/CCM.0000000000001483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 Supplementary Figure 1. Fluid assessment in the first seven days after time zero; A) fluid input, B) fluid output, C) fluid balance.
Data Availability Statement
The data used for this research are available from the corresponding author on reasonable request and subject to Institutional Review Board guidelines.