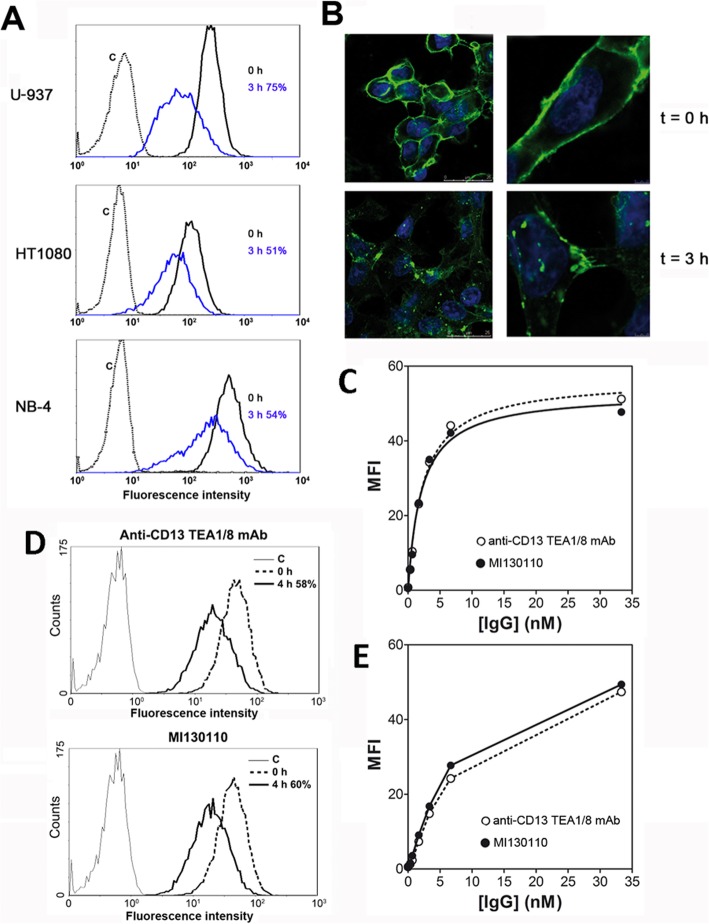
Fig. 2.
Cellular uptake of TEA1/8 and MI130110. a HT1080, U-937, and NB-4 cells (1E06) kept in suspension were incubated with TEA1/8 (10 μg/mL) for 3 h at 37 °C. Cells were then washed with cold PBS, labeled with rabbit anti-mouse FITC on ice for 30 min, and analyzed by flow cytometry. Cells labeled with isotype control were used as negative control. The percentage of CD13 endocytosis was calculated as the decrease of MFI of CD13 staining after 3 h incubation at 37 °C relative to the CD13 MFI at t = 0. b HT1080 cells (1E05) were plated in complete tissue culture medium onto poly-lysinated cover glasses and allowed to settle for 24 h at 37 °C and 5% CO2. Then, cells were left untreated or incubated with 5 μg/mL TEA1/8 for 3 h at 37 °C. Cells were fixed with 1:1 (v/v) methanol/acetone, washed, and labeled with rabbit anti-mouse FITC. Nuclei were visualized by DAPI staining. Figure shows untreated cells (t = 0), and cells allowed to internalize CD13 for 3 h (t = 3h). Samples were analyzed by confocal microscopy. Scale bars are shown. c HT1080 cells (1E06) were incubated with the indicated concentrations of TEA1/8 or MI130110 for 30 min on ice. After washing, cells were labeled with rabbit anti-mouse FITC and analyzed by flow cytometry. The resulting data were used to calculate the binding affinities of both molecules to CD13 by non-linear regression fitting of the experimental data to a classical binding isotherm equation considering one class of binding sites, the curves shown in the graph correspond to such regression. d HT1080 cells (1E06) were incubated in suspension with 10 μg/mL of either TEA1/8 or MI130110 at 37 °C for 4 h. Once washed, cells were labeled with rabbit anti-mouse FITC and analyzed by flow cytometry. Cells labeled with isotype control were used as negative control. The percentage of endocytosis at 4 h was calculated as the decrease of the MFI of CD13 staining after 4 h incubation at 37 °C relative to the CD13 MFI at t = 0. e HT1080 cells (1E06) were incubated in 200 μL culture medium with the indicated concentrations of TEA1/8 or MI130110 for 12 h at 37 °C to allow endocytosis to proceed. Then, 100 μL of the supernatants were harvested and used to stain CD13 on fresh HT1080 cells (1E06) as described for c. Concentrations in the horizontal axis correspond to those initially used in the first incubation