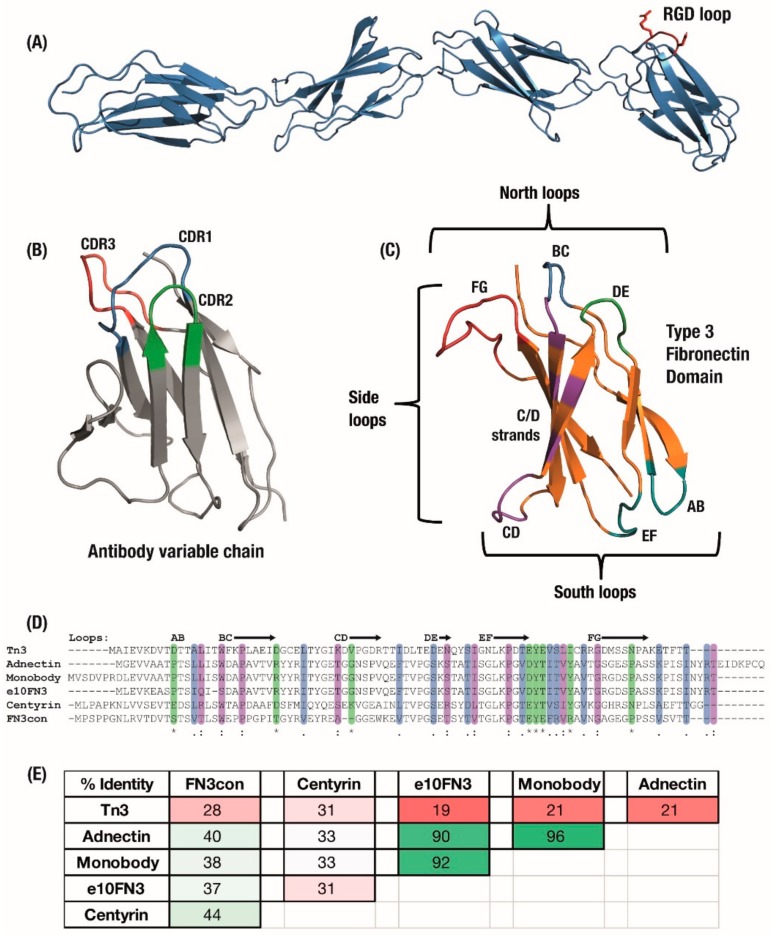
Figure 1.
(A) The Type 3 Fibronectin domains 7 to 10 from human fibronectin with the original RGD binding sequence highlighted in red. (B) Antibody domains use a set of three hypervariable binding loops to form a complementary region to a target binding site. (C) Fibronectin type III (FN3) domains have a comparable set of analogous loops which can be engineered for similar binding function, as well as an expanded binding footprint in the side and ‘south’ loops. Six derivatives of the FN3 domain under development have similar size and structure but can vary widely in (D) amino acid sequence, sharing only the F-Strand sequence across the domains, which leads to (E) a large variation in overall sequence identity between derivatives. Colouring: (D) Sequence alignment: */green—identical amino-acid,:/purple—strongly similar,/blue—weakly similar.; (E) Sequence pairwise identity matrix: green—highly identical sequences, pale green—strongly identical sequences, pink—weakly identical, red—low identical amino-acid matches.