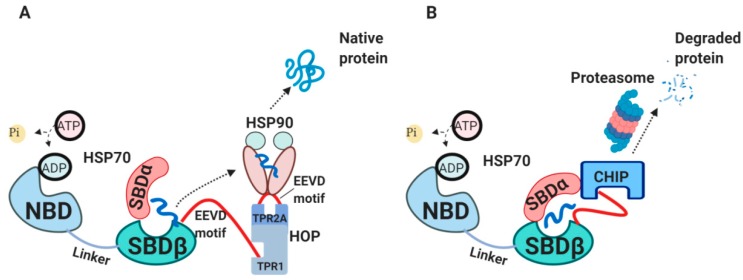
Figure 2.
The external HSP70 network. (A) HSP70-HSP90-HOP complex. HOP acting as an adaptor molecule mediates the association between HSP70 and HSP90. HSP70 C-terminal EEVD motif binds TPR1 on HOP, whereas HSP90 C-terminal EEVD binds TPR2A [49]. This association via HOP allows handing over the substrate from HSP70 to HSP90 for further folding. (B) HSP70-CHIP complexes. CHIP binds to C-terminus of HSP70-substrate complex as well as to SBDα lid through its TPR domain, ubiquitylates HSP70-bound peptides and targets them for proteasomal degradation [55,57]. HOP, HSP70/HSP90-organizing protein; TPR, tetratrico-peptide repeats; CHIP, C-terminus of HSP70 interacting protein.