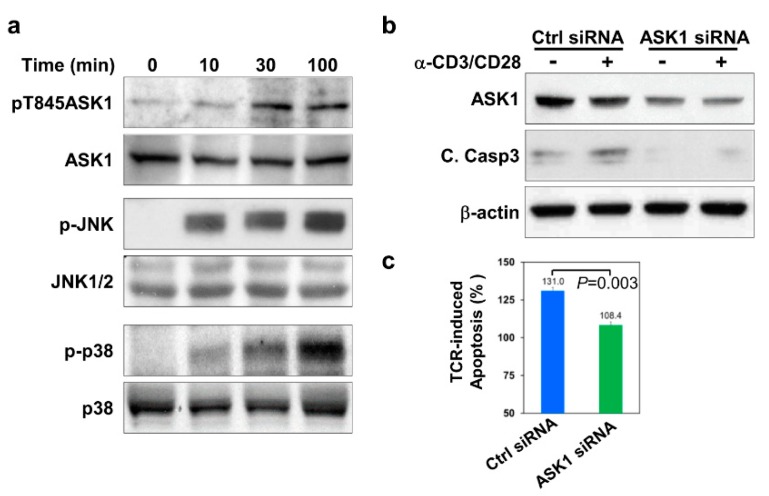
Figure 1.
ASK1 is activated and involved in the apoptosis of thymocytes upon TCR stimulation. (a) Activation of ASK1 and JNK/p38 in thymocytes upon TCR-engagement. Thymocytes were stimulated with anti-CD3 (10 μg/mL) and anti-CD28 (1 μg/mL) antibodies for various periods of time, as indicated. Whole cell lysates were prepared and subjected to immunoblotting to examine the phosphorylation status of ASK1 at Thr845, JNK at Thr183/Tyr185, and p38 at Thr180/Tyr182, respectively. The amount of ASK1, JNK1/2, and p38 was also detected and included as loading controls for the individual protein. (b) Knockdown of ASK1 and its effect on TCR-induced caspase-3 activation in thymocytes. Thymocytes were transfected with control siRNA (ctrl siRNA) or ASK1-specific siRNA (ASK1 siRNA), followed by culture without or with anti-CD3 (10 μg/mL) and anti-CD28 (1 μg/mL) antibodies for 16 h. Whole cell lysates were prepared and subjected to immunoblotting for the detection of ASK1 and the activated or cleaved form of caspase-3. An anti-β-actin blot was included as the loading control. (c) Suppression of TCR-induced apoptosis of thymocytes by ASK1 knockdown. Thymocytes were transfected with ctrl siRNA or ASK1 siRNA and treated with anti-CD3 (10 μg/mL) and anti-CD28 (1 μg/mL) antibodies for 16 h. Cells were harvested and stained with Annexin V and propidium iodide (PI), followed by a flow cytometric analysis. The percentage of TCR-induced apoptosis of thymocytes was expressed as: (percentage of dead cells with TCR treatment - percentage of dead cells without TCR treatment)/percentage of dead cells without TCR treatment. The data shown are representative of more than three independent experiments (a,b). The bar graph is shown as the mean ± SD (n = 3).