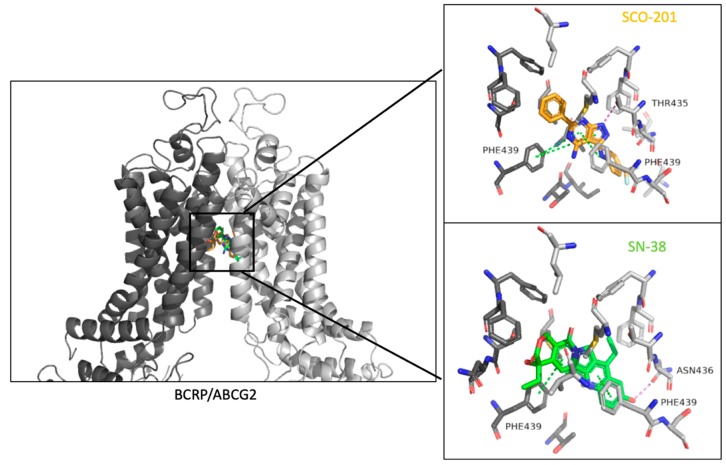
Figure 6.
Docking of SN-38 and SCO-201 in the human BCRP transporter. The transporter is a homomeric dimer with chain A (dark grey) and chain B (light grey). SN-38 (green) and SCO-201 (orange) bind in the same binding cavity when docked using Glide, Schrödinger, 2019-3, LLC [39,40,41]. Both the substrate SN-38 and the proposed inhibitor SCO-201 interacted with Phenylalanine (PHE)-439 in both chains of the protein through hydrophobic Pi-stacking interactions. SCO-201 formed a hydrogen bond to Threonine (THR)-435, whereas SN-38 formed a hydrogen bond to Aspargine (ASN)-436. Pi-stacking interactions are colored green and hydrogen bonds are colored violet.