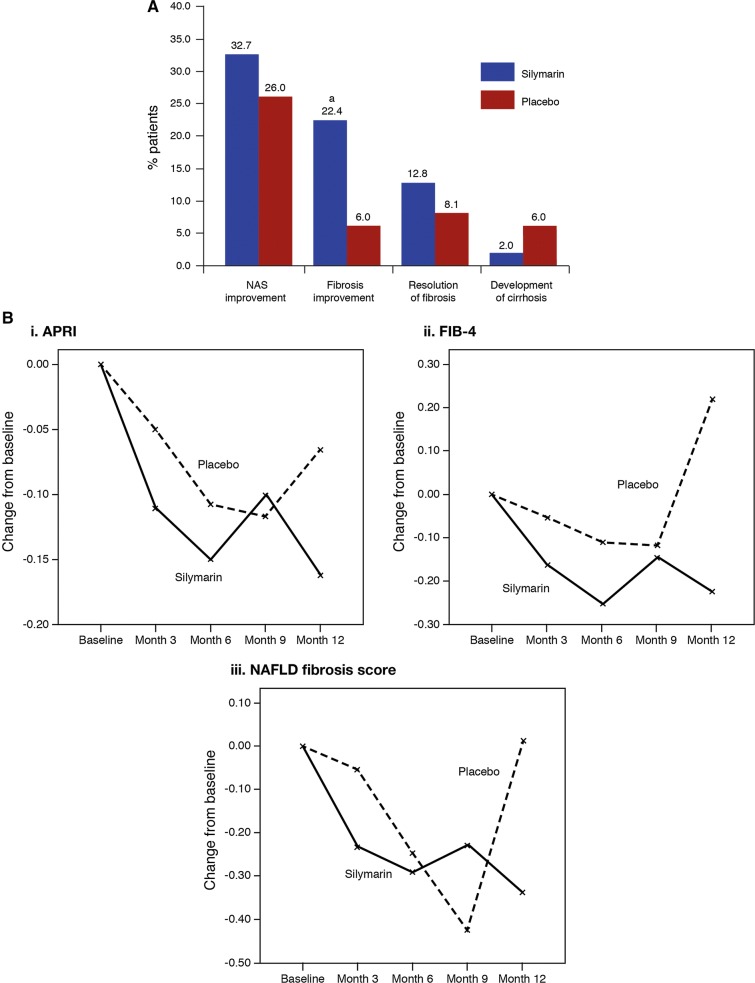
Fig. 5.
Effect of 48 weeks’ treatment with silymarin 2100 mg/day in 99 patients with histologically proven non-alcoholic steatohepatitis [79]. A proportion of patients with: a NAS score improvement (defined as ≥ 30% improvement in NAS), fibrosis improvement (defined as a ≥ 1 point improvement in the histologic component of the NAS score), resolution of fibrosis (defined as absence of fibrosis at EOT) or development of cirrhosis [79]. aP < 0.05 vs. placebo; bline charts illustrating the changes in the (i) APRI, (ii) FIB-4 score and (iii) NAFLD fibrosis scores in the silymarin and placebo groups [79]. APRI aspartate aminotransferase to platelet ratio index, EOT end of treatment, FIB-4 fibrosis-4, NAFLD nonalcoholic fatty liver disease, NAS NASH and NAFLD activity score, NASH non-alcoholic steatohepatitis.
Part B of figure reproduced with permission from Wah Kheong et al. [79]