Abstract
Background:
Colorado tick fever (CTF) is an underreported tick-borne viral disease occurring in the western United States. CTF illness includes fever, headache, and severe myalgia lasting for weeks. Wyoming has one of the highest CTF incidence rates with approximately 30% of infected persons reporting tick exposure in a Wyoming National Park or Forest before symptom onset. We assessed CTF virus infections among humans and Dermacentor andersoni ticks in Grand Teton National Park (GRTE) and Bridger-Teton National Forest (BTNF).
Methods:
In June of 2010, 526 eligible employees were approached to participate in a baseline and 3-month follow-up serosurvey and risk behavior survey. Seropositivity was defined as antibody titers against CTF virus ≥ 10, as measured by the plaque reduction neutralization test. Ticks were collected at 27 sites within GRTE/BTNF and tested by RT-PCR for the CTF virus.
Results:
A total of 126 (24%) employees participated in the baseline and follow-up study visits. Three (2%) employees were seropositive for CTF virus infection at baseline. During the study, 47 (37%) participants found unattached ticks on themselves, and 12 (10%) found attached ticks; however, no participants seroconverted against CTF virus. Walking through sagebrush (p = 0.04) and spending time at ≥ 7000 feet elevation (p < 0.01) were significantly associated with tick exposure. Ninety-nine percent (174/176) of ticks were D. andersoni, and all were found at ≥ 7000 feet elevation in sagebrush areas; 37 (21%) ticks tested positive for CTF virus and were found at 10 (38%) of 26 sites sampled.
Conclusions:
Although no GRTE or BTNF employees were infected with CTF virus during the study period, high rates of infected ticks were identified in areas with sagebrush at ≥ 7000 feet. CTF education and personal protection measures against tick exposure should be targeted to visitors and employees traveling to the high-risk environs identified in this study.
Keywords: Wyoming, Colorado tick fever, Dermacentor andersoni
Introduction
Colorado tick fever (CTF) is caused by the CTF virus, a RNA virus that is transmitted by Dermacentor andersoni (Florio et al. 1946, Florio et al. 1950, Marfin and Campbell 2005). CTF virus distribution mirrors that of its tick vector, which is endemic in mountainous regions of the western United States and southwestern Canada at elevations of 4000–10,000 feet above sea level (Emmons 1988, Romero and Simonsen 2008). After the bite of an infected tick, the incubation period for clinical illness is typically 2–3 days (Goodpasture et al. 1978). The majority of symptomatic patients have a nonspecific illness with a fever that is often biphasic, headache, chills, and myalgia (Spruance and Bailey 1973, Goodpasture et al. 1978). Approximately 20% of patients are hospitalized (Goodpasture et al. 1978, Brackney et al. 2010). Symptoms can last for weeks, with fatigue and weakness often persisting for months following acute disease (Earnest et al. 1971, Goodpasture et al. 1978). No specific treatment for the disease exists, and clinical management is primarily supportive. Because of its nonspecific symptoms, CTF is believed to be frequently misdiagnosed and under-diagnosed (Brackney et al. 2010).
Reported cases of CTF have declined dramatically since the 1980s. During 1988–1998, a median of 65 cases/year were reported to the Centers for Disease Control and Prevention (CDC), whereas during 1999–2007, a median of 14 CTF cases/year were reported (range, 2–88/year) (Brackney et al. 2010, CDC unpublished data 2010). Although some of the decrease might be the result of changing ecology and human risk behaviors, some is likely caused by changes in reporting practices (e.g., Colorado removing CTF from its reportable disease list in 1997).
CTF is a reportable disease in Wyoming within 24 h of diagnosis. During 1990–2008, a median of three cases of CTF (range, 0–7) were reported annually to the Wyoming Department of Health (WDH). Of these cases, 69% had disease onset in June or July, and 30% reported a tick exposure in one of Wyoming’s national parks or forests before disease onset. During 2009, Wyoming reported nine cases of CTF, the highest number of cases reported to CDC by a state since 1998 (Marfin and Campbell 2005, CDC unpublished data 2010). All cases were in Sublette County for a county incidence of 88 cases/100,000 persons (WDH unpublished data 2011).
Bridger-Teton National Forest (BTNF) and Grand Teton National Park (GRTE) are contained within and around Sublette County. Given their location in a region with high CTF incidence, the numerous employees and visitors who spend time outdoors in these areas are likely at increased risk for CTF virus infection. However, no studies have been conducted in these areas regarding tick distribution and CTF viral burden or virus transmission risk, particularly among park and forest employees. This study aimed to estimate CTF viral burden among collected D. andersoni ticks in areas frequented by visitors and employees, assess tick exposures and prevention practices among employees, and estimate the prevalence of CTF virus exposure among GRTE and BTNF employees.
Materials and Methods
Study sites
BTNF abuts GRTE and serves as a gateway for GRTE visitors. BTNF employs > 100 full-time and seasonal staff and has > 2 million visitors annually (BTNF unpublished data 2010). It covers 485 square miles with an elevation range of 6350–13,770 feet (US Department of Agriculture, undated); however, the majority of trails and areas frequented by visitors are located at ≥ 7000 feet. GRTE is one of the most visited US National Parks (> 4 million visitors/year). The park employed 426 persons during 2009 (GRTE unpublished data 2010). It covers 5353 square miles with an elevation range of 5629–13,804 feet (Uhler 1995–2007). In contrast with BTNF, the majority of commonly visited areas and trails in GRTE are located below 7000 feet.
Study population
Eligible study participants included all full-time employees of GRTE (n = 426) and BTNF (n = 100) aged ≥ 18 years who were expecting to remain at their current employment location for a minimum of 3 months (June–August 2010). Employees were recruited to participate in the study through informational flyers posted throughout the park and distributed through employee e-mails. This study was conducted with approval from and in compliance with the standards of WDH’s and CDC’s institutional review committees, and written consent was obtained from all participants.
Tick exposure survey and blood collection
At the baseline visit conducted during the first week of June, 2010, we collected demographic and occupational data and collected a serum sample. At the 3-month follow-up visit conducted in August, we administered a paper-based survey to assess knowledge, attitudes, and practices regarding tick-borne disease, and collected exposure data on ticks and tick habitat. We obtained data on both the number of ticks found unattached on the skin or clothes and ticks found attached on the skin. For the purpose of this study, tick exposure was defined as one or more attached or unattached tick(s) found on a participant during the study period. Finally, a serum sample was also collected during the follow-up visit in August, 2010.
Tick collections
Collection sites were selected throughout GRTE and BTNF and mapped with a global positioning system. Site selection was based on trails and campsites that are heavily used and, when possible and accessible, to areas containing previously described tick habitat, particularly those at ≥ 7000 feet (US Armed Forces Pest Management Board 1998, Eisen et al. 2008). Ticks were collected on three occasions from late May to early August by using methods previously described (Eisen 2007). To estimate tick density, tick drags consisting of a 1 × 1.25-meter flannel cloth were examined for ticks every 15 sec, 120 times/site, yielding a total of 90 sampling min/tick-sampling site. Ticks were collected from the drags and staff’s clothing, transferred to vials, flash frozen on dry ice, and transported to the Arboviral Diagnostic Laboratory at the CDC in Fort Collins, CO.
CTF virus testing and interpretation
At each study visit, blood was collected from participants and shipped by overnight courier to the Wyoming Public Health Laboratory. Samples were spun down to obtain sera, labeled with unique study identification numbers, and stored at – 70°C before being shipped to the CDC on dry ice. At the CDC, human sera samples were tested for CTF virus–specific neutralizing antibodies by 90% plaque reduction neutralization test (PRNT), as previously described (Calisher et al. 1985). Seropositivity was defined as serum antibody titers ≥ 10. Collected ticks were thawed and sorted by species and sex, enumerated, and tested for CTF virus presence by RT-PCR by using standardized methods previously described (Lambert et al. 2007, Brown et al. 2011).
Data analysis
Data were entered into an Epi Info™ (CDC, Atlanta, GA) database. We defined a previous CTF virus infection as a seropositive result at baseline and less than four-fold change in neutralizing antibody titer between baseline and follow-up serum samples. We defined an acute CTF virus infection as either seroconversion between negative and positive neutralizing titers or a four-fold or greater change in neutralizing antibody titer between baseline and follow-up serum samples.
An entomologic risk index (ERI), a measure of the average number of infected ticks to which a person might be exposed in tick habitat, was calculated using methods similar to those previously described (Mather et al. 1996). More specifically, we calculated the ERI as the product of D. andersoni tick density (average number of ticks collected per minute) and the proportion of CTF virus–infected D. andersoni ticks. We took a conservative approach by calculating the ERI for D. andersoni females only, as female ticks are more likely to feed repeatedly and thus are more likely to transmit CTF virus to humans (Klompen et al. 1996).
Demographic and behavioral characteristics of participants were compared by using the Fisher exact or Pearson chi-squared test; p values < 0.05 were considered statistically significant. All reported p values are two-sided. Statistical analysis of data was conducted by using StatXact® (Cytel, Inc., Cambridge, MA), and SAS® v.9.1 (SAS Institute Inc., Cary, NC).
Results
Serosurvey enrollment, and participant demographic characteristics
Of the 526 eligible GRTE/BTNF employees, 158 (30%) enrolled in the study, and 126 (24%) returned for the required 3-month follow-up assessment (Fig. 1). Of the 126 total employees completing both study visits, 106 were from GRTE and 20 were from BTNF. The median age of study participants was 35 years (range, 19–76 years); 77 (61%) were male and 121 (96%) were white. The distribution of age, sex, and race did not differ significantly between eligible and enrolled employees. The median number of years study participants had worked at GRTE/BTNF was 1.1 (range, 0.3–27 years); 89% of participants began working on or before May 1, 2010 (Table 1).
FIG. 1.
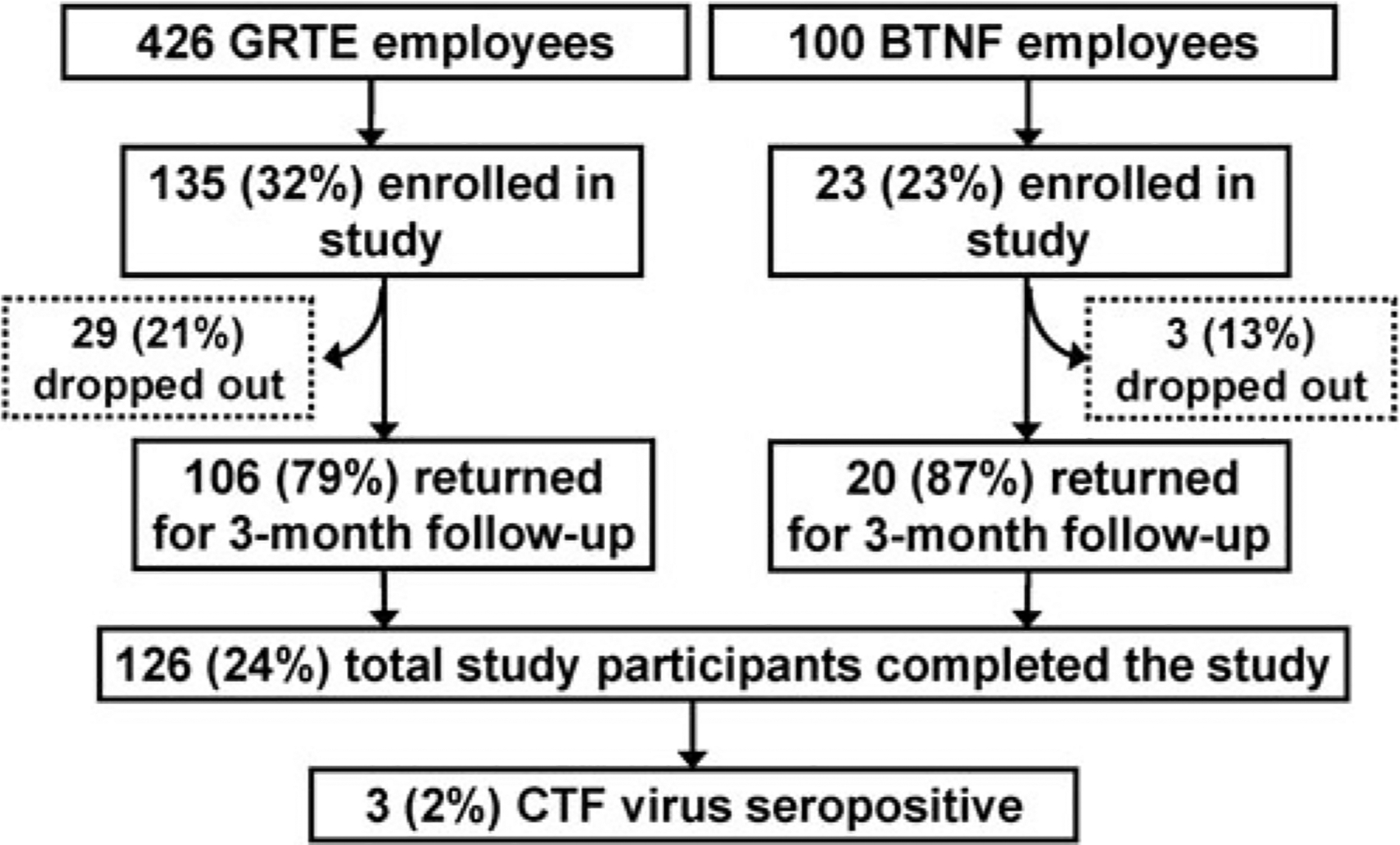
Enrollment and serologic results for Colorado tick fever (CTF) study, Grand Teton National Park (GRTE) and Bridger-Teton National Forest (BTNF), June–August, 2010.
Table 1.
Demographic Characteristics of Serosurvey Participants, Grand Teton National Park (GRTE) and Bridger-Teton National Forest (BTNF), June–August, 2010a
| GRTE | BTNF | Total | ||||
|---|---|---|---|---|---|---|
| n = 106 | n = 20 | n = 126 | ||||
| Characteristic | No. | (%) | No. | (%) | No. | (%) |
| Sex | ||||||
| Male | 64 | (60) | 13 | (65) | 77 | (61) |
| Race | ||||||
| White | 100 | (94) | 19 | (95) | 119 | (94) |
| Asian | 1 | (1) | 1 | (5) | 2 | (2) |
| Black | 1 | (1) | 0 | (0) | 1 | (1) |
| Other/Unknown | 4 | (4) | 0 | (0) | 4 | (3) |
| Age (years) | ||||||
| Median (range) | 35 | (21–76) | 41 | (19–62) | 35 | (19–76) |
| Employed at site (years) | ||||||
| Median (range) | 1 | (0.3–26) | 2 | (0.3–27) | 1 | (0.3–27) |
| Worked outdoors | ||||||
| > 20 h/week | 60 | (57) | 14 | (70) | 74 | (59) |
| Tick historyb | ||||||
| None | 67 | (63) | 12 | (60) | 79 | (63) |
| ≥ 1 unattached | 39 | (37) | 8 | (40) | 47 | (37) |
| ≥ 1 attached | 11 | (10) | 1 | (5) | 12 | (10) |
Only those who attended both study visits and completed the risk factor survey are included (n = 126).
Tick exposure was defined as one or more ticks found on a person during June–August, 2010.
GRTE, Grand Teton National Park; BTNF, Bridger-Teton National Forest.
Tick exposure risks and tick prevention measures
The majority of employees (59%) reported working outdoors > 20 hours/week (Table 1). The vast majority (85%) of employees had weekly exposures to sagebrush and spent time at elevations ≥ 7,000 feet; smaller proportions reported walking off-trail, handling brush, sitting on or leaning against logs and trees, performing fence repair, or spending time in picnic areas and campgrounds. Approximately 50% of employees reported having experienced five or more of these exposures per week (Table 2).
Table 2.
Risk Factors for Tick Exposures Among Serosurvey Participants, Grand Teton National Park and Bridger-Teton National Forest, June–August, 2010
| Survey question | Tick exposurea | |||
|---|---|---|---|---|
| Yes | No | |||
| During your workweek, do you … | n/N (%) | n/N (%) | Relative riskb | 95% CI |
| Go > 7000 ft elevation? | 46/106 (43) | 0/16 (0) | ∞ | (2.2−∞) |
| Walk in sagebrush? | 46/116 (40) | 0/7 (0) | ∞ | (1.1−∞) |
| Sit on logs/lean on trees? | 45/103 (44) | 1/18 (6) | 7.9 | (1.2–53.5) |
| Walk off trails? | 44/109 (40) | 1/13 (8) | 5.2 | (1.2–80.5) |
| Handle leaves/brush? | 40/83 (48) | 6/38 (16) | 3.1 | (1.4–6.6) |
| Walk in campgrounds? | 39/95 (41) | 5/24 (21) | 2.0 | (0.9–4.5) |
| Perform fence repair? | 15/33 (45) | 27/83 (33) | 1.4 | (0.9–2.3) |
| Wear insect repellent? | 25/66 (38) | 22/60 (37) | 1.0 | (0.6–1.6) |
Tick exposure was defined as one or more ticks found on a person during June–August, 2010.
Relative risk was calculated using statXact®and represents the risk of a particular activity or measure in the group exposed to ticks divided by the risk of the same activity or measure in the group not exposed to ticks.
CI, confidence interval.
In total, 47/126 (37%) participants reported a tick exposure during the 3-month study. All 47 reported finding one or more unattached ticks on themselves during the study period, and 12 (26%) also had one or more attached ticks during the study period (Table 1). Walking off-trail was identified as a risk factor for tick exposure, with 40% of those reporting walking off-trail having tick exposure, compared with only 8% of participants who did not walk off-trail (relative risk [RR], 5.2; 95% confidence interval [CI] 1.2–80.5; p = 0.02). Handling leaves, brush, or wood and sitting on logs or leaning against trees were significantly associated with tick exposure (RR = 3.1; 95% CI 1.4–6.6; p < 0.01; and RR = 7.9; 95% CI 1.2–53.5; p < 0.01, respectively). Walking in sagebrush (p = 0.04) and travelling at elevations ≥ 7000 feet (p < 0.01) also were significantly associated with tick exposure. Performing fence repair and walking in picnic areas and campgrounds where visitors frequented were not associated with tick exposure (Table 2).
Of the 126 participants, 121 (96%) responded to questions regarding everyday use of personal protective measures. The majority of respondents (71%) reported wearing long pants, whereas long sleeves were worn by only 33% (n = 40) of employees, and only 3% (four) reported tucking their pants into socks or boots. Fifty-four (45%) of 121 employees reported conducting tick checks of skin and clothing > 50% of the time after working outdoors; 27 (22%) reported never conducting tick checks. All 126 participants responded to questions regarding repellent use; 87 (69%) reported having used insect repellant at least once since entry into the study. However, only 66 (52%) used repellant at work and even fewer, 20 (16%), reported using repellant > 50% of the time. We determined no statistical difference in tick exposure between those who reported having used insect repellent during the work week and those who did not.
Serologic testing results
Of the 126 study participants providing baseline and follow-up serum samples, three (2%) tested seropositive at baseline and did not have a four-fold or greater change in their titers between their samples, indicating a previous exposure to CTF virus. None of the study participants seroconverted against CTF virus during the 3-month study period; therefore, no participants were classified as having recent CTF infection. All of the 32 study participants who were lost to follow-up had tested negative for CTF virus antibodies at baseline.
Of the three persons with previous CTF virus exposure, two were employees at GRTE, and one at BTNF. All participants with previous exposure to CTF virus were white men, with an age range of 20–50 years. Furthermore, all were residents of a state where CTF is endemic. They had all worked at GRTE/BTNF for > 2 years, and two of three worked outdoors for > 20 h/week. None had previously received a CTF diagnosis.
Tick survey
Tick surveys were conducted at 12 sites in BTNF and 14 in GRTE. All (12/12) of the sites in BTNF were ≥ 7000 feet elevation, but only 14% (2/14) of drag sites in GRTE were at ≥ 7000 feet. In BTNF, 174 adult ticks were found at 9 (75%) of the sites sampled. All ticks were D. andersoni and found at elevations ≥ 7000 feet. Of the 174 ticks, 37 (21%) tested positive for CTF virus. In GRTE, two adult Dermacentor albopictus, a tick that can carry CTF virus but rarely bites humans, were found at one of 14 sites. Both of these ticks were found at 6800 feet elevation and were negative for CTF virus by RT-PCR.
We sampled 12 BTNF sites for 0.32 sample hours each during three visits, totaling 11.6 sample hours. Of the 174 D. andersoni ticks we collected, 102 were female, giving a female tick encounter rate of 8.8 females/hour. The infection rate for females was 23% (23/102), resulting in an ERI of 2.0 infected female ticks/hour for BTNF. An ERI was not calculated for GRTE because only two D. albopictus ticks were collected and both were negative for CTF virus.
Discussion
This is one of the first studies to document the potential risk and burden of CTF virus in Wyoming’s national parks and forests. BTNF and GRTE employ a considerable number of employees and are visited by millions of people each year, all of whom potentially are exposed to tick-borne diseases. Approximately one-third of participating park employees found a tick on themselves during the 3-month study duration. Risk factors for tick exposure in our study included walking through sagebrush, spending time at elevations ≥ 7000 feet, handling leaves and brush, and walking off-trail. At least two of these risk factors, sagebrush presence and elevations of ≥ 6890 feet, also had been identified in a previous study to be indicators of elevated risk for tick exposures (Eisen et al. 2007, 2008).
CTF virus-infected D. andersoni were found in BTNF at elevations ≥ 7000 feet, with approximately two infected ticks encountered/hour and 200 CTF virus–infected ticks/1000 ticks collected. Our number of infected ticks per collection is roughly twice the rates reported previously in studies conducted in Colorado, including studies conducted in Rocky Mountain National Park (McLean et al. 1981, Eads and Smith 1983, Brown et al. 1989). The higher rates in our study might be attributed to use of a more sensitive RT-PCR, compared with previous studies that used Vero cell culture plaque assays and mouse infection studies to identify virus presence.
Despite the relatively high prevalence of infected ticks in our study and more than one-third of employees reporting exposures to ticks, none of the surveyed employees demonstrated signs of seroconverting against the virus during the study period. However, 2% of participants were seropositive for CTF virus at baseline, indicating prior infection. This finding is consistent with a recent study that identified 8% (5/60) of employees at Rocky Mountain National Park in Colorado with preexisting CTF virus antibodies, but also found none of the employees experienced incident CTF virus infection, despite one-third reporting tick exposure during the authors’ 1-year study period (Adjemian et al. 2012). Although studies have confirmed that tick infection rates are correlated with incidence of tick-borne diseases, they also found that this must be paired with human behavior measures to be useful in predicting risk for tick-borne disease (Connally et al. 2006).
Tick prevention measures are well-established; however, of the tick prevention measures assessed in our study, only wearing long pants, a standard uniform component, was practiced > 50% of the time by employees. Although roughly half of participants in our study reported using repellants at least once during a work week, only 16% reported using it > 50% of the time. This is consistent with the findings reported by Adjemian et al., where 44% of employees reported using repellent at work. Interestingly, we determined that the majority of employees conduct tick checks after spending time outdoors. These results, combined with our finding that 75% of employees reporting a tick exposure had unattached ticks on them, indicate that employees might be removing the ticks before CTF virus transmission can occur. Therefore, emphasizing to both employees and visitors the importance of checking for ticks to prevent tick attachment and disease transmission might be a useful prevention message for decreasing the risk for CTF virus infection.
One limitation of our study is that GRTE vector surveillance was restricted by site selection because we selected sites most visited by GRTE visitors and employees to obtain an estimate of tick exposure for the majority of visitors rather than sites that were > 7000 feet and difficult to access. This likely resulted in an underestimate of D. andersoni prevalence and potential CTF virus infection rate and of the risk to persons hiking or working at higher elevation and in areas that are more ideal tick habitats. Additional tick sampling is needed at GRTE locations ≥ 7000 feet where employees reported tick exposure to better estimate the entomologic risk in these areas. The ERI calculated for BTNF is conservative and does not take into account potential bites from male ticks, which if included would increase the encounter rate to 15 ticks encountered/hour and the ERI to 3.0 CTF virus-infected ticks/hour. In addition, the ERI only estimates the number of ticks encountered, not the number of ticks that might attach, and only applies to the sites sampled in this study. Our 30% participation rate of park and forest employees makes generalization of our findings to the entire cohort difficult. Our limited sample size and high prevalence of tick habitat exposures precluded a multivariable analysis of risk for tick exposures. If an employee acquired an early-season (April–May) CTF infection prior to our June baseline visit, they would have been misclassified as having a past infection in our study. Finally, we were unable to analyze risk factors for CTF virus infection because of a lack of new CTF cases in our study.
The national parks and forests provide a unique environment where persons are recreating in tick-endemic areas and thus are at risk for multiple tick-borne diseases (Adjemian et al. 2012). In addition to CTF virus, D. andersoni is a vector for two additional known pathogens that cause Rocky Mountain spotted fever and tularemia (McLean et al. 1993, Treadwell et al. 2000, Hayes 2005). Given this, physicians should consider tick-borne diseases among employees or travelers who become ill while or after being in Wyoming’s national parks or forests. In addition, employees should be provided information and guidance regarding tick-borne diseases endemic to the park and forest, areas likely to harbor ticks, personal protection measures against tick exposure (www.cdc.gov/ticks/avoid), and importance of prompt and proper removal of ticks. Our findings can be used to inform public health interventions for park and forest visitors, but further studies are needed to assess visitor risk for tick-borne diseases.
Acknowledgments
This research was funded by the Centers for Disease Control and Prevention and the Wyoming Department of Health. The funders had a role in study design, data collection and analysis, decision to publish, and manuscript preparation.
The authors thank Lars Eisen (Colorado State University) for his technical assistance in tick-collection methodology. We express our gratitude for the countless efforts and contributions of the CDC staff, WDH field and laboratory assistants, and Wyoming public health nurses who made this project possible, and special thanks to Kris Bisgard, Kay Smith, Katherine Gibney, Katie Bryan, Marc Fischer, Reginald McClinton, Seth Mathern, Ashley Busacker, Amber Erickson, Charles Hall, Daniel O’Leary, Kelly Weidenbach-Vigil, Brandon Johnson, Jim Walford, Danielle Stafford, Courtney Craigan, Tammy Marshall, Sandra Marshall-Goodson, Jeff Eason, Connie Sweeney, and Dereth Gehl-hausen. We gratefully acknowledge the incredible assistance from the staff of both Grand Teton National Park and Bridger-Teton National Forest, including Sue Consolo-Murphy, Michael Colombe, and Jose Castro.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Provisional findings were presented at the 60th Epidemic Intelligence Service Conference in 2011, Atlanta, GA, and at the Council of State and Territorial Epidemiologists Annual Conference in 2011, Pittsburgh, PA.
References
- Adjemian J, Weber IB, McQuiston J, Griffen KS, et al. Zoonotic infections among employees from Great Smoky Mountains and Rocky Mountain National Parks, 2008–2009. Vector Borne Zoonotic Dis 2012; 12:922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackney MM, Marfin AA, Staples JE, Stallones L, et al. Epidemiology of Colorado tick fever in Montana, Utah, and Wyoming, 1995–2003. Vector Borne Zoonotic Dis 2010; 10:381–385. [DOI] [PubMed] [Google Scholar]
- Brown HE, Yates KF, Dietrich G, MacMillan K, et al. An acarologic survey and Amblyomma americanum distribution map with implications for tularemia risk in Missouri. Am J Trop Med Hyg 2011; 84:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SE, Miller BR, McLean RG, Knudson DL. Co-circulation of multiple Colorado tick fever virus genotypes. Am J Trop Med Hyg 1989; 40:94–101. [DOI] [PubMed] [Google Scholar]
- Calisher CH, Poland JD, Calisher SB, Warmoth LA. Diagnosis of Colorado tick fever virus infection by enzyme immunoassays for immunoglobulin M and G antibodies. J Clin Microbiol 1985; 22:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connally NP, Ginsberg HS, Mather TN. Assessing peridomestic entomological factors as predictors for Lyme disease. J Vector Ecol 2006; 31:364–370. [DOI] [PubMed] [Google Scholar]
- Eads RB, Smith GC. Seasonal activity and Colorado tick fever virus infection rates in Rocky Mountain wood ticks, Dermacentor andersoni (Acari: Ixodidae), in north-central Colorado, USA. J Med Entomol 1983; 20:49–55. [DOI] [PubMed] [Google Scholar]
- Earnest MP, Breckinridge JC, Barr RJ, Francy DB, et al. Colorado tick fever: Clinical and epidemiologic features and evaluation of diagnostic methods. Rocky Mt Med J 1971; 68:60–62. [PubMed] [Google Scholar]
- Eisen L Seasonal pattern of host-seeking activity by the human-biting adult life stage of Dermacentor andersoni (Acari: Ixodidae). J Med Entomol 2007; 44:359–366. [DOI] [PubMed] [Google Scholar]
- Eisen L, Meyer AM, Eisen RJ. Climate-based model predicting acarological risk of encountering the human-biting adult life stage of Dermacentor andersoni (Acari: Ixodidae) in a key habitat type in Colorado. J Med Entomol 2007; 44:694–704. [PubMed] [Google Scholar]
- Eisen L, Ibarra-Juarez LA, Eisen RJ, Piesman J. Indicators for elevated risk of human exposure to host-seeking adults of the Rocky Mountain wood tick (Dermacentor andersoni) in Colorado. J Vector Ecol 2008; 33:117–128 [DOI] [PubMed] [Google Scholar]
- Emmons RW. Ecology of Colorado tick fever. Annu Rev Microbiol 1988; 42:49–64. [DOI] [PubMed] [Google Scholar]
- Florio L, Stewart MO, Mugrage ER. The etiology of Colorado tick fever. J Exp Med 1946; 83:1–10. [PMC free article] [PubMed] [Google Scholar]
- Florio L, Miller MS, Mugrage ER. Colorado tick fever; isolation of the virus from Dermacentar andersoni in nature and a laboratory study of the transmission of the virus in the tick. J Immunol 1950; 64:257–263. [PubMed] [Google Scholar]
- Goodpasture HC, Poland JD, Francy DB, Bowen GS, et al. Colorado tick fever: Clinical, epidemiologic, and laboratory aspects of 228 cases in Colorado in 1973–1974. Ann Intern Med 1978; 88:303–310. [DOI] [PubMed] [Google Scholar]
- Hayes EB. Tularemia In: Goodman JL, Dennis DT, Sonenshine DE, eds. Tick-Borne Diseases of Humans. Washington, DC: ASM Press; 2005:207–217. [Google Scholar]
- Klompen JS, Black WC 4th, Keirans JE, Oliver JH Jr. Evolution of ticks. Annu Rev Entomol 1996; 41:141–161. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Kosoy O, Velez JO, Russell BJ, et al. Detection of Colorado tick fever viral RNA in acute human serum samples by a quantitative real-time RT-PCR assay. J Virol Methods 2007; 140:43–48. [DOI] [PubMed] [Google Scholar]
- Marfin AA, Campbell GL. Colorado tick fever and related coltivirus infections In: Goodman LJ, Dennis DT, Sonenshine DE, eds. Tick-Borne Diseases of Humans. Washington, DC: ASM Press, 2005:143–149. [Google Scholar]
- Mather TN, Nicholson MC, Donnelly EF, Matyas BT. Entomologic index for human risk of Lyme disease. Am J Epidemiol 1996; 144:1066–1069. [DOI] [PubMed] [Google Scholar]
- McLean RG, Francy DB, Bowen GS, Bailey RE, et al. The ecology of Colorado tick fever in Rocky Mountain National Park in 1974. I. Objectives, study design, and summary of principal findings. Am J Trop Med Hyg 1981; 30:483–489. [DOI] [PubMed] [Google Scholar]
- McLean RG, Carey AB, Kirk LJ, Francy DB. Ecology of porcupines (Erethizon dorsatum) and Colorado tick fever virus in Rocky Mountain National Park, 1975–1977. J Med Entomol 1993; 30:236–238. [DOI] [PubMed] [Google Scholar]
- Romero JR, Simonsen KA. Powassan encephalitis and Colorado tick fever. Infect Dis Clin North Am 2008; 22:545–559. [DOI] [PubMed] [Google Scholar]
- Spruance SL, Bailey A. Colorado tick fever: A review of 115 laboratory confirmed cases. Arch Intern Med 1973; 131:288–293. [PubMed] [Google Scholar]
- Treadwell TA, Holman RC, Clarke MJ, Krebs JW, et al. Rocky Mountain spotted fever in the United States, 1993–1996. Am J Trop Med Hyg 2000; 63:21–26. [DOI] [PubMed] [Google Scholar]
- Uhler JW. Grand Teton National Park G. [Jackson, WY: ]: Hillclimb Media, 1995–2007. Available at www.grand.teton.national-park.com/info.htm. Accessed October 29, 2013. [Google Scholar]
- US Department of Agriculture (USDA) Forest Service. Bridger-Teton National Forest. Washington, DC: USDA, [page undated]. Available at www.fs.usda.gov/btnf. Accessed October 29, 2013. [Google Scholar]
- US Armed Forces Pest Management Board (AFPMB). Technical Guide No. 26 Tick-Borne Diseases: Vector Surveillance and Control. June, 1998.


