The chloroplast outer envelope membrane protein PLASTID DIVISION2 (PDV2) and its paralog PDV1 modulate assembly and remodeling, respectively, of the dynamic DRP5B ring to guide chloroplast division.
Abstract
Chloroplasts divide by binary fission, which is driven by a ring-like multiprotein complex spanning the inner and outer envelope membranes (OEMs) at the division site. The cytosolic DYNAMIN-RELATED PROTEIN 5B (DRP5B/ARC5) is a mechanochemical GTPase involved in binary fission of the chloroplast membrane in Arabidopsis (Arabidopsis thaliana), but the dynamics of its interactions with the chloroplast membranes and their regulation by guanine nucleotides and protein effectors remain poorly characterized. Using an Arabidopsis phot2 mutant with defects in chloroplast photorelocation movement, we determined that the ring structures of DRP5B at the chloroplast division site underwent subunit exchange with a cytosolic DRP5B pool. Mutant DRP5B proteins with impaired GTPase activity retained the ability to self-assemble at the constriction sites of chloroplasts, but did not rescue the chloroplast division defects in the Arabidopsis drp5B mutant. Our in vivo kinetic measurements of the DRP5B mutant T82D suggested that turnover of the DRP5B ring at the chloroplast division site is coupled to GTP hydrolysis. Furthermore, we established that DRP5B targeting to the chloroplast surface and assembly into a ring structure at the division site are specifically determined by the chloroplast outer OEM protein PLASTID DIVISION2 (PDV2), and that DRP5B-OEM dissociation is mainly mediated by PDV1, a paralog of PDV2. Thus, this study suggests that the mechanochemical properties of DRP5B on the chloroplast surface are dynamically regulated by its GTPase activity and major binding partners.
Chloroplasts are plant-specific organelles surrounded by double membranes that conduct photosynthesis as well as fatty acid and amino acid biosynthesis (Postbeittenmiller et al., 1992; Lopez-Juez and Pyke, 2005). The continuity of chloroplasts during cell division and reproduction is maintained by the division of preexisting chloroplasts (Possingham and Lawrence, 1983). Chloroplasts divide via FtsZ-dependent binary fission, which is driven by a ringlike chloroplast division machinery assembled by a multiprotein complex that spans the inner and outer envelope membranes (IEM and OEM, respectively) at the center of the chloroplast (Kuroiwa et al., 1998; Yoshida et al., 2012; Osteryoung and Pyke, 2014). DYNAMIN-RELATED PROTEIN 5B (DRP5B/ARC5), a cytosolic component of the chloroplast division machinery, is thought to function in the fission of chloroplast envelopes, as revealed by studies of the Arabidopsis (Arabidopsis thaliana) accumulation and replication of chloroplasts5 (arc5) mutant, which exhibits enlarged, dumbbell-shaped chloroplasts in mesophyll cells (Robertson et al., 1996; Gao et al., 2003; Hong et al., 2003).
Dynamin and dynamin-related proteins (DRPs) are mechanochemical GTPases that function via oligomerization and GTP-dependent conformational changes that exert forces on membranes, thereby promoting membrane fission (van der Bliek and Meyerowitz, 1991; Ford et al., 2011; Mears et al., 2011; Ferguson and De Camilli, 2012). Dynamin has a low affinity for nucleotides and a high rate of GTP hydrolysis; it also self-assembles, forming higher-order structures such as rings and spirals on a lipid template (Stowell et al., 1999; Klockow et al., 2002). Classical dynamins generally contain five conserved domains: an N-terminal GTPase domain that binds and hydrolyzes GTP, a middle region involved in self-assembly and oligomerization, a lipid-interacting pleckstrin homology (PH) domain, a GTPase effector domain that is also involved in self-assembly, and a Prorich domain (Ford et al., 2011). The cytosolic DRP5B, a member of the dynamin superfamily, exhibits GTPase activity and can self-interact (Gao et al., 2003; Holtsmark et al., 2013). During chloroplast division, DRP5B subunits must be recruited from the cytosol to the chloroplast surface and assemble into a ring-like structure (DRP5B ring) at the chloroplast division site, facilitating fission of the chloroplast envelope (Gao et al., 2003). However, the molecular nature of DRP5B-membrane interactions and their regulation by guanine nucleotides and protein effectors remain poorly characterized.
In Arabidopsis, PLASTID DIVISION1 (PDV1) and its paralog PDV2 are key components of the chloroplast division machinery in the OEM (Miyagishima et al., 2006). Both proteins span the lipid bilayer via a single transmembrane domain and localize into ring-like structures at the chloroplast division site (Miyagishima et al., 2006; Glynn et al., 2008). ARC6 and PARALOG OF ARC6 (PARC6) are chloroplast division components in the IEM that directly interact with PDV2 and PDV1, respectively, in the intermembrane space, resulting in the positioning of PDV2 and PDV1 at the center of chloroplasts (Glynn et al., 2008, 2009; Zhang et al., 2016). The N termini of PDV1 and PDV2, which reside in the cytosol, interact with each other, and the cytosolic region of PDV1 interacts with the DRP5B PH domain in yeast two-hybrid assays (Holtsmark et al., 2013). It has been thought that PDV1 and PDV2 have redundant functions in DRP5B recruitment and the regulation of its GTPase activity (Miyagishima et al., 2006; Holtsmark et al., 2013). However, this assertion is inconsistent with the observation that the loss-of-function mutants pdv1 and pdv2 exhibit chloroplast division defects that appear similar to that of drp5B (Gao et al., 2003; Miyagishima et al., 2006). Therefore, it remains uncertain whether PDV1 and PDV2 have distinct roles in regulating DRP5B’s behavior on the cytosolic surface of the OEM during chloroplast division.
Here, fluorescence recovery after photobleaching (FRAP) analysis of chloroplasts showed that the DRP5B ring at the chloroplast division site undergoes subunit exchange during chloroplast membrane constriction, and that DRP5B-OEM dissociation is driven by GTP hydrolysis in vivo, possibly reflecting DRP5B’s biochemical mechanism of action in chloroplast envelope remodeling. Furthermore, our findings suggested that PDV2 and PDV1 favor DRP5B-OEM association and dissociation, respectively, at the chloroplast division site.
RESULTS
DRP5B Ring Undergoes Subunit Exchange at the Chloroplast Division Site
DRP5B exhibits GTP hydrolysis activity in vitro (Fig. 1A; Holtsmark et al., 2013), but the behavior of DRP5B on the chloroplast membrane in the presence of GTP, a more physiologically relevant condition, has not been investigated. Therefore, we conducted FRAP analysis of assembled and constricted DRP5B rings on the chloroplast surface at the division site. We fused the genomic DRP5B with the 3′ end of GFP and transformed this construct, GFP-DRP5Bg, into drp5B-1 (Ler background) and wild-type (ecotype Columbia of Arabidopsis [Col-0]) plants. The chloroplast division defect in drp5B-1 was rescued by GFP-DRP5Bg (Fig. 1, B and C), indicating that this GFP-tagged DRP5B fusion protein was fully functional.
Figure 1.
FRAP analysis of GFP-DRP5B dynamics on phot2-1 chloroplasts. A, GTPase activity of DRP5B in vitro. The time course of GTP hydrolysis by GST-DRP5B (100 nm) was measured in 1 mm GTP at 23°C. Pi, Inorganic phosphate. These data represent the average of at least three independent experiments with multiple independently purified batches of protein, and results are presented as the mean ± sd. B, Chloroplast morphology in living mesophyll cells of the indicated lines. C, Quantitative analysis of chloroplast number versus mesophyll cell size in 3-week-old seedlings. n = 50. D, Time-lapse images showing chloroplast movement in mesophyll cells of the indicated transgenic lines using 488-nm laser excitation to observe GFP and chlorophyll fluorescence. GFP and chlorophyll were falsely colored green and magenta, respectively. The time points of image acquisition are shown at the top. Dotted yellow lines serve as position references. E, Immunoblot analysis of GFP-DRP5Bg levels in the indicated plants. Actin served as a loading control. F, FRAP analysis of GFP-DRP5Bg dynamics on chloroplasts in living and fixed mesophyll cells of transgenic phot2 plants. The images show fluorescent signals in photobleached regions (circled areas) just before bleaching (Prebleach), at the time of bleaching (0 s), at the time closest to 50% recovery time ( t1/2; 120 s), and at 3 × t1/2 (360 s). The plots at right show normalized fluorescence recovery (Norm. fluor. inten.) versus time. Dotted lines are outlines of constricted chloroplasts.
Time-lapse confocal laser-scanning microscopy showed that the 488-nm laser excitation that was used to observe fluorescent signals from GFP-DRP5Bg and chlorophyll induced chloroplast movement in the mesophyll cells of transgenic wild-type plants (Fig. 1D), reminiscent of the finding that blue light induces chloroplast movement in mesophyll cells (Kagawa et al., 2001). We therefore tried to perform FRAP analysis of GFP-DRP5Bg in the phot2-1 (Col-0 ecotype) mutant, which lacks the UV-A/blue light receptor PHOT2. The phot2-1 mutant exhibits severe defects in chloroplast photorelocation due to impaired regulation of chloroplast-actin filaments (Kong et al., 2013). In the phot2-1/GFP-DRP5Bg plants, chloroplast number and size were similar to those of the wild type and wild-type/GFP-DRP5Bg plants, indicating that the loss of function of PHOT2 did not affect chloroplast division (Fig. 1, B and C). Confocal time-lapse imaging showed that, in contrast to transgenic wild-type plants, transgenic phot2-1 plants exhibited attenuated chloroplast movement during the laser scans (Fig. 1D).
FRAP experiments using mesophyll chloroplasts of transgenic phot2-1 plants with levels of GFP-DRP5Bg similar to those of transgenic wild-type plants (Fig. 1E) revealed fluorescence recovery of chloroplastic GFP-DRP5Bg in the bleached regions (Fig. 1F; Supplemental Video S1), indicating that the DRP5B rings on chloroplasts undergo subunit exchange with a cytosolic pool of DRP5B. To ensure that the cells were alive, we selected mesophyll cells containing visible GFP-DRP5Bg patches in the cytosol for the FRAP experiments. As a negative control, we determined that fluorescence recovery of chloroplast-localized GFP-DRP5Bg was not detected in glutaraldehyde-fixed leaf cells from transgenic phot2 plants after photobleaching (Fig. 1F, bottom). Together, these results indicate that mesophyll cells of transgenic phot2-1 plants could be successfully used to analyze GFP-DRP5Bg dynamics on chloroplasts and demonstrate that the DRP5B ring assembled at the chloroplast division site undergoes active subunit turnover.
DRP5B GTPase Activity Mutants K61A and T82D Still Localize at the Constriction Sites of Enlarged Chloroplasts in drp5B
The most highly conserved region of dynamin superfamily proteins (DSPs) is the GTPase domain (Marks et al., 2001). For dynamin, the mutations K44A and T65A/D in the GTPase domain both impair its GTPase activity (Damke et al., 1994; Warnock et al., 1996; Marks et al., 2001; Song et al., 2004). The residues that correspond to dynamin’s K44 and T65 in DRP5B are K61 and T82 (Fig. 2A). Arabidopsis DRP5B encodes two functional isoforms of 777 amino acids (hereafter referred to as DRP5B) and 741 amino acids (the short variant of DRP5B) as a result of alternative splicing in its PH domain (Gao et al., 2003). Glutathione S-transferase (GST)-tagged DRP5B encoded by the full-length DPR5B complementary DNA (cDNA) exhibited GTPase activity, hydrolyzing GTP in vitro (Fig. 1A). To test whether DRP5B GTPase activity is affected by the K61A and T82D mutations, we separately introduced each mutation into the DRP5B GTPase domain and purified the resulting GST-DRP5B(K61A) and GST-DRP5B(T82D) proteins from bacterial cells under native conditions (Fig. 2B). GTP hydrolysis activity of DRP5B was reduced in both mutants (Fig. 2C), consistent with what was predicted based on the analysis of conserved amino acids in DSPs (Fig. 2A).
Figure 2.
Functional analyses of GTPase active-site mutants of DRP5B. A, K61A and T82D mutations introduced into the GTPase domain of DRP5B. DRP5B K61 and T82 in the GTPase domain are conserved among dynamin superfamily members, as indicated by the alignment of a segment of the GTPase domain from the dynamin homologs DRP5B (GenBank: NP_188606.2), ScDnm1 (Uniprot: CAA97444.1), HsDmn1(Uniprot: P50570.2), and HsDmn2 (Uniprot: Q05193.2). Sc, Saccharomyces cerevisiae; Hs, Homo sapiens. B, Purified GST-DRP5B, GST-DRP5B(K61A), GST-DRP5B(T82D), and GST only for GTPase activity analysis, separated on a Coomassie blue-stained SDS-PAGE gel. BSA, Bovine serum albumin, loaded as a protein concentration standard; WT, wild type. C, GTPase activities of DRP5B active-site mutants K61A and T82D. One hundred fifty nanomoles of each protein were measured in 1 mm GTP at 23°C. These data represent the average of three independent experiments, and results are presented as the mean ± sd. Pi, Inorganic phosphate. D, The drp5B-2 mutant is fully complemented by the GFP-tagged full-length cDNA DRP5B. E and F, Both GFP-DRP5B(K61A) (E) and GFP-DRP5B(T82D) (F) localize into ring structures at the constriction sites of enlarged chloroplasts, but neither of them rescues the drp5B-2 mutants. White arrowheads indicate the ring structures of GFP-DRP5B(K61A) and GFP-DRP5B(T82D) at the constriction sites of enlarged chloroplasts. In D to F, GFP and chlorophyll were falsely colored green and magenta, respectively. G, Immunoblot analysis of protein levels of GFP-DRP5BL(K61A) or GFP-DRP5B(T82D) in the individual transgenic lines. Actin served as a loading control.
For consistency, we fused GFP with DRP5B encoded by the full-length cDNA and found that in vivo, this GFP-DRP5B construct fully complemented the drp5B-2 mutation in the Col-0 background (Fig. 2D). We next generated the constructs GFP-DRP5B(K61A) and GFP-DRP5B(T82D) and transformed them individually into the transfer DNA (T-DNA) insertion mutant drp5B-2. We analyzed the chloroplast morphologies and localization patterns of GFP-DRP5B(K61A) and GFP-DRP5B(T82D) in the mesophyll cells of four individual T1 transgenic plant lines of each type (Fig. 2, E and F). Like wild-type GFP-DRP5B, both K61A and T82D mutant fusion proteins assembled into ring structures at the constriction sites of enlarged chloroplasts, but neither of them rescued the chloroplast division defects in the transgenic drp5B-2 mutant (Fig. 2, D–F). Immunoblot analysis of different individual T1 transgenic lines revealed that accumulation levels of GFP-DRP5B(K61A) and GFP-DRP5B(T82D) were similar to that of GFP-DRP5B (Fig. 2G). These results indicate that DRP5B targeting to and self-assembly at the chloroplast division site are not impaired by the GTPase active-site mutations K61A and T82D.
Exchange Kinetics of DRP5B at the Chloroplast Division Site Depend on GTP Hydrolysis
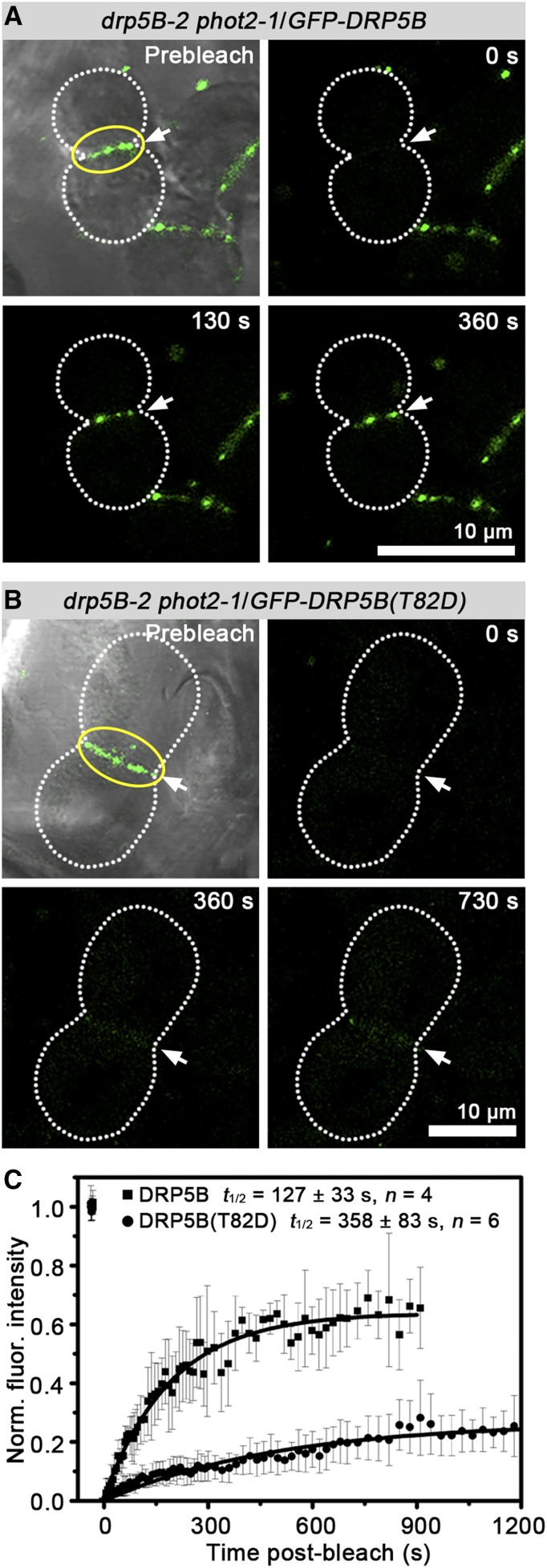
To investigate whether impaired GTPase activity affects the mobility of DRP5B in the ring structures at the constriction sites of chloroplasts, we next quantified FRAP in plants of the transgenic drp5B-2 phot2-1 background in which endogenous DRP5B was replaced with GFP-tagged DRP5B or its active-site mutant T82D. After photobleaching, the turnover rate of DRP5B tested at the chloroplast division site was 127 ± 33 s (mean ± sd, n = 4; Fig. 3A; Supplemental Video S2). In contrast, the average half-life (t1/2) of DRP5B(T82D) recovery on chloroplasts was 358 ± 83 s (n = 6; Fig. 3B; Supplemental Video S3). Turnover of DRP5B(T82D) was markedly slower than that of wild-type DRP5B (Fig. 3C), indicating that the dynamics of DRP5B on the chloroplast surface is essential for chloroplast membrane constriction. In vitro, GTP hydrolysis activity was impaired in the T82D mutant (Fig. 2C), reminiscent of the reports demonstrating that the dynamin T65A/D substitution attenuates GTP hydrolysis in vitro (Marks et al., 2001; Song et al., 2004). Hence, direct comparison of turnover rates of DRP5B and DRP5B(T82D) rings on the chloroplast surface suggest that GTP hydrolysis might elicit a conformational change of DRP5B in the assembled ring structures that favors DRP5B dissociation from the chloroplast OEM.
Figure 3.
FRAP analysis of the GFP-DRP5B(T82D) ring at the chloroplast constriction sites in the drp5B-2 phot2-1 background. A, FRAP analysis of the GFP-DRP5B ring on the dividing chloroplasts. B, FRAP analysis of GFP-DRP5B(T82D) dynamics at the constriction sites of enlarged chloroplasts. In A and B, the chloroplast was photobleached at the location indicated by the arrow, and the fluorescence recovery in the circled region was monitored over time. Images were photographed just before bleaching (Prebleach) and then at the times indicated. Dotted lines show outlines of the constricted chloroplasts. C, Comparison of recovery of fluorescence in DRP5B and DRP5B(T82D). Normalized fluorescence recovery (Norm. fluor. intensity) is plotted versus time. The average t1/2 is given as the mean ± sd. n indicates the number of individual GFP-DRP5B or GFP-DRP5B(T82D) rings analyzed in mesophyll cells.
PDV2 Recruits Cytosolic DRP5B to the Chloroplast Division Site, Facilitating DRP5B Ring Assembly
Key molecular mechanisms for chloroplast division include the recruitment of cytosolic DRP5B to the chloroplast and the subsequent assembly and remodeling of the DRP5B ring at the division site. These steps might be mediated by receptors, adapters, or regulators located in the OEM. To date, only two OEM proteins, PDV1 and PDV2, have been shown to function in chloroplast division (Miyagishima et al., 2006). Here, we observed that PDV1 and PDV2 colocalized with DRP5B during the early, middle, and late stages of chloroplast division (Fig. 4, A and B). This observation, combined with the finding that DRP5B interacted with both PDV1 and PDV2 in a bimolecular fluorescence complementation (BiFC) assay (Supplemental Fig. S1; Holtsmark et al., 2013), suggests that PDV1 and PDV2 might affect DRP5B activity at the chloroplast division site throughout the division process.
Figure 4.
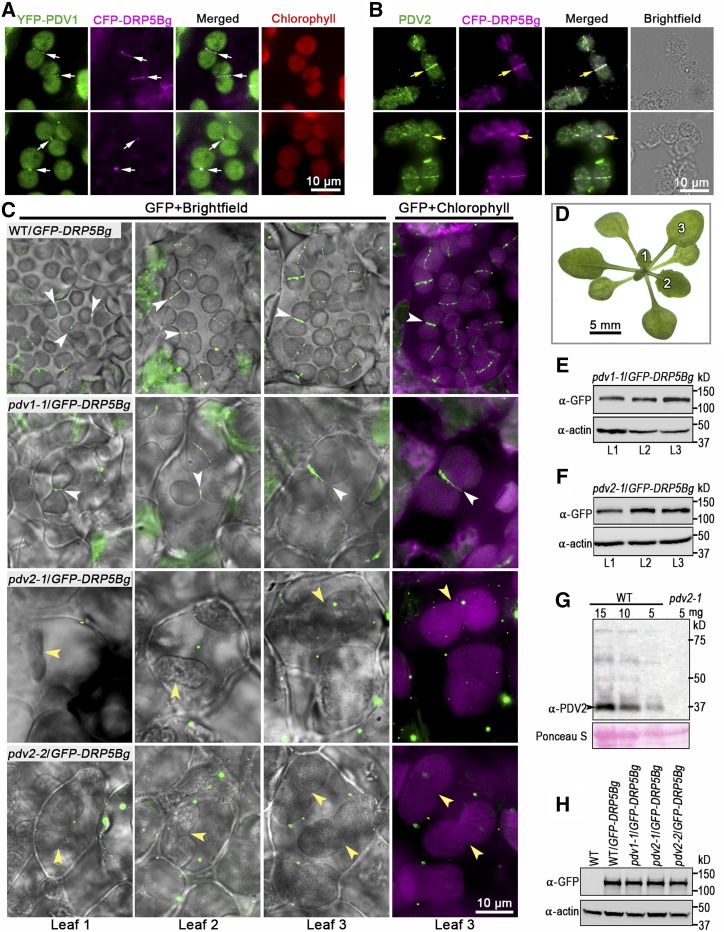
Localization patterns of GFP-DRP5Bg in Arabidopsis pdv1 and pdv2 mutants. A, YFP-PDV1 and CFP-DRP5Bg fluorescence in living mesophyll cells of transgenic wild-type plants. The signals from YFP, CFP, and chlorophyll were falsely colored green, magenta, and red, respectively. White arrows indicate that CFP-DRP5Bg and YFP-PDV1 signals were simultaneously detected at the chloroplast division site. B, Colocalization of PDV2 with CFP-DRP5Bg examined by dual immunofluorescence microscopy. Fluorescent signals from Alexa Fluor 594-labeled PDV2 and 488-labeled CFP-DRP5Bg were falsely colored green and magenta, respectively. Yellow arrows indicate that immunostained-DRP5B and PDV2 were simultaneously detected at the chloroplast division site. C, GFP-DRP5Bg localization patterns in mesophyll cells from the indicated transgenic plants. GFP and chlorophyll signals were falsely colored green and magenta, respectively. White arrowheads indicate GFP-DRP5Bg localized to ring-like structures, whereas yellow arrowheads indicate chloroplast membrane constriction sites lacking GFP-DRP5B signals. D, Numbers 1–3 indicate the rosette leaves of ∼3-week-old Arabidopsis plants analyzed by microscopy in C. E and F, GFP-DRP5Bg levels in leaves labeled 1–3 in C in the indicated transgenic plants. Actin served as a loading control. G, Immunoblot analysis showing that anti-PDV2 antibody detected a specific band at ∼37 kD in protein samples from the wild type (Col-0). This specific band was not detected in protein samples from pdv2-1. Ponceau S staining of Rubisco on the blot is shown as a loading control. H, GFP-DRP5Bg levels detected by GFP antibody in the indicated transgenic plants. Actin served as a loading control.
To assess DRP5B activity in the absence of PDV1 and PDV2, we needed to first examine the localization patterns of GFP-DRP5Bg in pdv1-1 and pdv2-1. As observed in transgenic wild-type plants, in transgenic pdv1-1 plants, GFP-DRP5Bg formed a ring structure at enlarged, constricted chloroplasts (Fig. 4C, first two rows). By contrast, no GFP-DRP5Bg was detected at the constriction sites of dumbbell-shaped chloroplasts in transgenic pdv2-1 plants (Fig. 4C, third row). These results are in conflict with a previous finding that in both pdv1-1 and pdv2-1, GFP-DRP5Bg localizes to chloroplast constriction sites (Miyagishima et al., 2006). The T-DNA in pdv2-1 is in an intron, so it seemed possible that it was partially spliced out in some plants or cells; therefore, we carefully examined the precise localization patterns of GFP-DRP5Bg in various leaves of 3-week-old transgenic pdv1-1 and pdv2-1 seedlings (Fig. 4D). Specific GFP-DRP5Bg bands were detected in protein extracts from different leaves of transgenic pdv1-1 and pdv2-1 upon immunoblotting using an anti-GFP antibody (Fig. 4, E and F). Unlike in transgenic pdv1-1 plants, GFP-DRP5Bg signals were invariably detected in the cytosol rather than at the constriction sites of enlarged chloroplasts during leaf development in the transgenic pdv2-1 plants (Fig. 4, C and D). We also did not detect endogenous PDV2 accumulation using a specific anti-PDV2 antibody in the pdv2-1 plants (Fig. 4G), suggesting that pdv2-1 was a null mutation. Additionally, the localization patterns of cyan fluorescent protein (CFP)-tagged DRP5B in transgenic pdv1-1 and pdv2-1 plants were the same as those of GFP-DRP5Bg: CFP-DRP5Bg localized to the constriction sites of enlarged chloroplasts in pdv1-1 but not pdv2-1 plants (Supplemental Fig. S2).
Our observations in pdv2-1 are inconsistent with the previous finding (Miyagishima et al., 2006), and the reason that different localization patterns of GFP-DRP5Bg were observed in the same pdv2-1 mutant is currently unclear. Another allele of pdv2, pdv2-2, which bears a T-DNA insertion in the second exon, has a phenotype similar to that of pdv2-1, with large, centrally constricted chloroplasts (Miyagishima et al., 2006). However, in pdv2-2, GFP-DRP5Bg was still detected in the cytosol rather than at the constriction sites of enlarged chloroplasts (Fig. 4C, bottom row). Furthermore, immunoblot analysis showed that the levels of GFP-DRP5Bg in transgenic wild-type, pdv1-1, pdv2-1, and pdv2-2 seedlings were similar (Fig. 4H), indicating that the absence of DRP5B at the constriction sites of enlarged chloroplasts was not due to a deficiency of GFP-DRP5Bg accumulation in these transgenic pdv2-1 and pdv2-2 plants. The observation of similar localization patterns of GFP-DRP5Bg in pdv2-1 and pdv2-2 plants strongly suggests that the loss of function of PDV2 blocks the targeting of DRP5B to the chloroplast surface, thereby preventing the assembly of the DRP5B ring at the division site.
PDV1 Affects Turnover of the DRP5B Ring at the Chloroplast Division Site
Chloroplast division is blocked in the pdv1 mutants, but PDV1 had no effect on DRP5B recruitment and assembly on the chloroplast surface (Fig. 4C; Miyagishima et al., 2006). Therefore, we performed in vivo FRAP analysis of the DRP5B rings in the pdv1-1 background to further assess the potential effect of PDV1 loss on DRP5B activity. FRAP analysis of GFP-DRP5Bg on 10 chloroplasts from phot2-1 plants revealed that the average t1/2 of GFP-DRP5Bg turnover at the chloroplast constriction sites was 116 ± 13 s (Fig. 5A). We next created transgenic pdv1-1 phot2-1 plants expressing GFP-DRP5Bg by genetic crossing, and performed FRAP analysis of GFP-DRP5Bg on enlarged, constricted chloroplasts in these plants. After photobleaching, fluorescence recovery of the DRP5B ring tested was not detected in the fixed mesophyll cells, but low recovery was observed in the living cells (Fig. 5B; Supplemental Video S4). The average t1/2 of fluorescence recovery of GFP-DRP5Bg rings was 322 ± 74 s in transgenic pdv1-1 phot2 plants (Fig. 5B, right; n = 9 chloroplasts), which is markedly slower than that in the transgenic phot2-1 plants (Fig. 5A). These differences were statistically significant (Student’s t test, P < 0.01), indicating that PDV1 plays a role in regulating DRP5B dynamics in the chloroplast division machinery.
Figure 5.
Kinetics of GFP-DRP5Bg rings on chloroplasts are affected by the loss of PDV1. A, FRAP analysis of GFP-DRP5B dynamics on the chloroplast surface in mesophyll cells of transgenic phot2-1 plants. B, FRAP analysis of GFP-DRP5Bg dynamics on chloroplasts in living and fixed mesophyll cells of transgenic pdv1-1 phot2-1 plants. The images show fluorescent signals in photobleached regions (circled areas) just before bleaching (Prebleach) and at the times indicated. Dotted lines show outlines of constricted chloroplasts. White arrowheads indicate the constriction sites of the chloroplast membrane. Fluorescent signals from GFP were falsely colored green. The plots at right show the corrected, normalized fluorescence intensity (Norm. fluor. intensity) as a function of time after photobleaching, yielding normalized average fluorescence recovery. Error bars represent the mean ± sd at each time point. n represents the number of individual GFP-DRP5Bg rings analyzed in mesophyll cells of each transgenic plant.
Amount of Available PDV2 Does Not Affect Turnover of the DRP5B Ring on the Chloroplast Surface
Since PDV2-1/pdv2-1 chloroplasts exhibit an abnormal phenotype intermediate between that of wild-type and pdv2-1 chloroplasts (Miyagishima et al., 2006), we also analyzed the localization of GFP-DRP5Bg in the heterozygous PDV2-1/pdv2-1 background. GFP-DRP5Bg accumulation was not altered (Fig. 6A), but endogenous PDV2 levels were apparently lower in transgenic PDV2-1/pdv2-1 than in transgenic wild-type plants (Fig. 6B). Despite this deficiency in PDV2 accumulation, GFP-DRP5Bg was still able to form rings at the constriction sites of abnormally enlarged chloroplasts during leaf development (Fig. 6, B and C). To assess the recovery rate of the GFP-DRP5Bg ring on chloroplasts in the PDV2-1/pdv2-1 background, we then created transgenic PDV2-1/pdv2-1 phot2-1 plants expressing GFP-DRP5Bg by genetic crossing, and performed FRAP analysis of the DRP5B ring on these abnormal chloroplasts. Fluorescence recovery of GFP-DRP5Bg was observed in living but not fixed mesophyll cells of 3-week-old plants (Fig. 6D; Supplemental Video S5), indicating that the DRP5B ring structures are dynamic even in the presence of reduced amounts of PDV2. We collected data from 10 individual FRAP analyses of GFP-DRP5Bg and found that the average t1/2 of GFP-DRP5Bg recovery was 122 ± 26 s (Fig. 6D; n = 10 chloroplasts). The DRP5B rings in the phot2-1 and PDV2-1/pdv2-1 phot2-1 backgrounds displayed statistically similar recovery kinetics (Figs. 5A and 6D; Student’s t test, P > 0.05), suggesting that the amount of available PDV2 does not affect the turnover rate of the dynamic DRP5B ring on the chloroplast surface.
Figure 6.
Dynamic DRP5B rings are observed in a heterozygous PDV2-1/pdv2-1 background. A, Immunoblot analysis of GFP-DRP5Bg levels in the indicated transgenic plants. Actin served as a loading control. B, Endogenous PDV2 levels in transgenic wild-type and PDV2-1/pdv2-1 plants examined by immunoblotting. Actin served as a loading control. C, GFP-DRP5Bg localization patterns in mesophyll cells from transgenic wild-type and PDV2-1/pdv2-1 plants. GFP and chlorophyll signals were falsely colored green and magenta, respectively. White arrowheads indicate GFP-DRP5Bg localized to ringlike structures at the chloroplast membrane constriction sites. D, FRAP analysis of GFP-DRP5Bg dynamics on chloroplasts in living and fixed mesophyll cells of transgenic PDV2-1/pdv2-1 phot2-1 plants. The images show fluorescent signals in photobleached regions (circled areas) just before bleaching (Prebleach), at the time of bleaching (0 s), at the time closest to t1/2 (120 s), and at 2 × t1/2 (360 s). The plots at right show normalized fluorescence recovery (Norm. fluor. inten.) versus time. The average t1/2 is shown as the mean ± sd. Dotted lines show outlines of constricted chloroplasts. Arrowheads indicate the GFP-DRP5Bg ring at the chloroplast constriction site. n represents the number of individual GFP-DRP5Bg rings analyzed in the mesophyll cells.
DISCUSSION
DRP5B, which belongs to a superfamily of mechanochemical GTPase enzymes, is reported to be involved in the division of chloroplasts, peroxisomes, and mitochondria (Robertson et al., 1996; Gao et al., 2003; Zhang and Hu, 2010; Aung and Hu, 2012). To date, however, high-order structures assembled by DRP5B, such as rings, have been clearly observed only on the chloroplast surface at the division site (Gao et al., 2003). In this study, we overcame the complication of chloroplast photorelocation movement by using the Arabidopsis phot2 mutant for FRAP analysis of the GFP-DRP5B ring on chloroplasts of mesophyll cells, and we determined that the chloroplast DRP5B ring is dynamic during membrane constriction (Fig. 1F), undergoing assembly and remodeling at the division site. To examine whether membrane-associated mechanochemical properties of DRP5B are directly coupled to its GTP activity, we analyzed mutant DRP5B proteins with impaired GTPase activity. Furthermore, we demonstrated that the chloroplast outer membrane proteins PDV1 and its paralog PDV2 differentially affect DRP5B-OEM interactions at the division site to promote chloroplast division. The distinct underlying mechanisms by which PDV1 and PDV2 promote membrane constriction at the chloroplast division site are illustrated in Figure 7.
Figure 7.
Schematic representation of the distinct roles of PDV1 and PDV2 in DRP5B-membrane interactions. A, Behavior of DRP5B on chloroplasts in the presence of OEM proteins PDV1 and PDV2. In wild-type mesophyll cells, the cytosolic GTPase DRP5B colocalizes with PDV1 and PDV2 at the chloroplast division site and assembles into a dynamic ring structure that undergoes GTP-dependent subunit exchange on the chloroplast surface. B and C, Behavior of DRP5B in the absence of either PDV2 (B) or PDV1 (C). The pdv1 and pdv2 mutants exhibit similar defects in chloroplast division, in which membrane constriction at the division site is blocked, resulting in a reduced number of chloroplasts with an enlarged, dumbbell shape in mesophyll cells; however, the underlying molecular mechanisms are intrinsically different. As the key components of the chloroplast division machinery in the OEM, PDV1 and PDV2 independently form ring structures at the division site. In pdv2, cytosolic DRP5B subunits cannot be recruited to the chloroplast surface, thereby preventing DPR5B assembly at the chloroplast division site (B), even though PDV1 still localizes to the membrane constriction site. By contrast, in pdv1, DRP5B assembles into a ringlike structure at the division site, but turnover of the DRP5B ring is impaired by the absence of PDV1 (C).
Like dynamin, DRP5B is a multidomain GTPase consisting of an N-terminal GTPase domain, a middle assembly domain, a PH domain, and a GTPase-effector domain (Gao et al., 2003; Hong et al., 2003; Ramachandran and Schmid, 2018). In vitro studies indicate that DRP5B harbors GTPase activity and can self-interact (Fig. 1A; Holtsmark et al., 2013). DRP5B mutants K61A and T82D, which reduced the GTP hydrolysis rate in vitro, retained the ability to target to the chloroplast surface and assemble into ring structures in vivo, but neither mutant elicited further constriction and fission of chloroplast envelopes at the division site (Fig. 2, C–F), suggesting that GTPase activity is required for DRP5B to drive chloroplast membrane fission in vivo. Our in vivo FRAP analysis revealed that the assembled GFP-DRP5B ring at the constriction site of a normal chloroplast undergoes subunit exchange with a cytosolic pool, continually remodeling itself with a half-time of ∼127 s in the absence of endogenous DRP5B (Fig. 3A). In contrast, FRAP kinetics of the mutant DRP5B(T82D) in the drp5B-2 phot2-1 background revealed a much slower turnover rate, consistent with the findings that K61A and T82D substitutions in DRP5B’s GTPase domain attenuated GTP hydrolysis in vitro (Figs. 2C and 3). In vitro, GTP hydrolysis causes the disassembly of dynamin spirals and the dissociation of dynamin subunits from the membrane (Song et al., 2004; Rahaman et al., 2008; Ramachandran and Schmid, 2008). Therefore, the slower turnover rate of DRP5B(T82D) in the assembled ring structures may in part reflect the inefficiency of this mutant DRP5B in dissociating from the OEM, suggesting that, like dynamin, DRP5B might mediate fission of the chloroplast membrane through a GTP-dependent conformational change on the OEM. Taken together, these in vivo analyses demonstrate that DRP5B’s mechanochemical properties on the surface of the chloroplast envelope are dynamically regulated by its GTPase activity. Thus, we propose that GTPase activity is required for DRP5B to facilitate chloroplast membrane remodeling at the division site.
The OEM protein PDV1 interacts and colocalizes with DRP5B at the center of chloroplasts during chloroplast division (Fig. 4A; Miyagishima et al., 2006; Holtsmark et al., 2013). Functional analyses of pdv1 mutants reveal that PDV1 functions as a positive regulator of chloroplast division (Miyagishima et al., 2006; Okazaki et al., 2009). In the absence of PDV1, DRP5B still forms a ring structure at the constriction sites of enlarged abnormal chloroplasts (Fig. 4C; Miyagishima et al., 2006), but the turnover rate of the DRP5B ring is reduced in response to the absence of PDV1 (Fig. 5), similar to what was seen for the DRP5B(T82D) mutant protein (Fig. 3). These findings suggest that the loss of PDV1 likely attenuates GTP hydrolysis or the disassembly capacity of the chloroplast DRP5B ring. However, a previous in vitro study showed that the cytosolic region of PDV1 (PDV1cyt) inhibits DRP5B’s GTPase activity (Holtsmark et al., 2013). If PDV1 also inhibited DRP5B GTPase activity in vivo, the turnover rate of the DR5PB ring should be faster in the absence than in the presence of PDV1, but that seems not to be the case. We noticed that the PDV1cyt used for assays of DRP5B’s GTPase activity in the previous study was purified under denaturing conditions (Holtsmark et al., 2013), so it is possible that in vitro-refolded PDV1cyt lacks the full function of the native PDV1. Alternatively, PDV1 might favor DRP5B remodeling on the surface of the chloroplast OEM via a direct effect on a conformational change of DRP5B. Overall, our in vivo analyses reveal that PDV1 likely facilitates chloroplast membrane fission by promoting DRP5B-OEM dissociation at the chloroplast division site.
PDV2, a paralog of PDV1, also functions as a positive effector promoting chloroplast division (Miyagishima et al., 2006; Glynn et al., 2008; Okazaki et al., 2009). Previous studies have suggested, based on analyses of DRP5B localization patterns in pdv1-1 and pdv2-1 single mutants and the pdv1-1 pdv2-1 double mutant, that PDV1 and PDV2 function redundantly to recruit the cytosolic DRP5B to the chloroplast surface at the division site (Miyagishima et al., 2006). Here, we examined DRP5B localization and found the same results as before in pdv1-1, showing that DRP5B was able to form a ring structure at the constriction sites of enlarged chloroplasts during leaf development (Fig. 4C; Miyagishima et al., 2006) and thus confirming that PDV1 has no effect on the process of DRP5B-OEM association in vivo. However, in pdv2-1 and pdv2-2 mutants, the signals from florescent-protein-tagged DRP5B were exclusively detected in the cytosol rather than as ring structures at the constriction sites of enlarged chloroplasts (Fig. 4, C and F; Supplemental Fig. S2). These radically distinct localization patterns of DRP5B in the pdv1-1 versus pdv2-1 or pdv2-2 mutants suggested that PDV1 and PDV2 have nonredundant roles in the association of DRP5B with the OEM and demonstrated that only PDV2 is essential for DRP5B recruitment to the chloroplast division site, facilitating DRP5B assembly on the chloroplast surface. Unlike PDV1, PDV2 exhibits semidominance, and PDV2-1/pdv2-1 heterozygotes display abnormal chloroplast phenotypes intermediate between those of the wild type and pdv2-1 (Miyagishima et al., 2006). Although PDV2-1/pdv2-1 plants had reduced levels of PDV2, DRP5B was still able to form a ring at the chloroplast constriction site (Fig. 6). However, the dynamics of the DRP5B ring at the division site was unaffected by the amount of available PDV2, as the recovery kinetics of the chloroplast DRP5B rings (i.e. t1/2) in the PDV2-1/pdv2-1 and PDV2-1/PDV2-1 backgrounds were not statistically different (Figs. 5A and 6D; Student’s t test, P > 0.05). Thus, our findings suggest that PDV2 may specifically function as a regulator of DRP5B-OEM association during chloroplast division.
In conclusion, our findings on the kinetics of the DRP5B ring and the differing effects of PDV1 and PDV2 on DRP5B-OEM interactions provide a further understanding of the underlying mechanism of action of the chloroplast division machinery in land plants. Chloroplasts are enclosed by a double-layered membrane that must coordinately constrict at the division site for chloroplast division to occur. This process requires the assembly of a multiprotein complex spanning the OEM and IEM at the division site. The cytoplasmic machinery comprises the dynamin-related protein DRP5B (Gao et al., 2003), whereas the endosymbiont-derived tubulin-like GTPase FtsZ forms the stromal apparatus (McAndrew et al., 2001; Vitha et al., 2001). At the stromal side, the IEM proteins ARC6 and PARC6 function in the assembly and disassembly, respectively, of the FtsZ ring (Vitha et al., 2003; Glynn et al., 2009; Zhang et al., 2016; Sung et al., 2018). Current findings suggest that the OEM proteins PDV2 and PDV1 function in different aspects of the assembly and remodeling/disassembly, respectively, of the DRP5B ring at the cytosolic surface of the chloroplast (Fig. 7). Among these four integral membrane proteins, ARC6 and PARC6 specifically interact with PDV2 and PDV1, respectively, in the intermembrane space (Glynn et al., 2008; Zhang et al., 2016; Wang et al., 2017). These findings suggest that during chloroplast division, the interaction between PDV2 and ARC6 might synchronize the assembly of DRP5B and FtsZ, whereas the interaction between PDV1 and PARC6 induces the disassembly of the ring-like structures of DRP5B and FtsZ to keep pace, causing the chloroplast envelopes to synchronously constrict at the division site.
MATERIALS AND METHODS
DNA Constructs
Sequences for the primer numbers below are supplied in Supplemental Table S1. The ∼2-kb 5′-flanking region of DRP5B was PCR amplified using primers A052/A053 and inserted into PstI/BamHI-digested pCAMBIA1300 or pCAMBIA3300, resulting in p1300-DRP5Bpro and p3300-DRP5Bpro, respectively. A CFP fragment without the stop codon and a 5,947-bp fragment of DRP5B genomic DNA were PCR-amplified using primers A003/A054 and A049/A050, respectively. Following KpnI digestion, the two fragments were ligated together and cloned into the T-vector (Clonesmarter). A BamHI/EcoRI-digested fragment of CFP-DRP5Bg was cloned into p1300-DRP5Bpro, generating the p1300-DRP5Bpro-CFP-DRP5Bg construct. After the CFP sequence in the T-CFP-DRP5Bg vector was replaced with GFP, the GFP-DRP5Bg fragment was cloned into p3300-DRP5Bpro, generating p3300-DRP5Bpro-GFP-DRP5Bg. To obtain PDV1pro-YFP-PDV1, the PDV1 promoter, YFP, and the coding region of PDV1 were combined and fused via splice overlapping PCR with primer sets C327/C328, C329/C330, and C331/C332, respectively (Warrens et al., 1997) and cloned into pCambia3300. For the GST-DRP5B construct, full-length DRP5B cDNA was PCR-amplified with primers C360/C361 and cloned into BamHI/SalI-digested pGEX-6p-1. A cDNA fragment containing the first 639 bp of the PDV2 coding region was amplified using primers C168/C020 and cloned into pHIS8-3. For the GFP-DRP5B construct, the fused fragments of GFP and the full-length DRP5B cDNA obtained using primers C152/145/146/C335 were digested with BglII and EcoRI and cloned into the p1300-DRP5Bpro. K61A and T82D mutations of DRP5B were generated by PCR-based mutagenesis using primer sets C312/C314 and C431/C433, respectively. All vectors were verified by sequencing.
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 (Col-0) was used as the wild-type control for genetic, transgenic, and phenotypic analyses. The Arabidopsis mutants used in this study were described previously, including phot2-1 (a G-to-A mutation at base 3,121; Kagawa et al., 2001), pdv1-1 (a G-to-A mutation at base 50; Miyagishima et al., 2006), and drp5B-1/arc5-1 (a G-to-A mutation at base 1,412; Marrison et al., 1999; Gao et al., 2003), and three T-DNA insertion lines, drp5B-2 (arc5-2, SAIL_71_D11), pdv2-1 (SALK_059656), and pdv2-2 (SAIL_875_E10; Miyagishima et al., 2006). With the exception of drp5B-1 in the Ler background, all other mutants are of the Col-0 ecotype. Arabidopsis Col-0, drp5B-1, or drp5B-2 plants harboring a transgene were generated by the floral dip method using Agrobacterium tumefaciens GV3101 (Clough and Bent, 1998). The wild-type plants coexpressing CFP-DRP5Bg and YFP-PDV1 and all other single or double mutants with a transgene were created by genetic crosses. Transgenic plants were selected on one-half strength Murashige and Skoog medium containing 30 μg/mL hygromycin B or 15 μg/mL glufosinate-ammonium. Then, 7-d-old seedlings were transplanted in soil and grown at 22°C and 60% relative humidity in broad-spectrum light (80 μmol photons m–2 s–1) with a 16 h light/8 h dark photoperiod.
GTPase Assays
GST and GST-tagged proteins were expressed in BL21 CodonPlus cells and purified under native conditions using a GST-affinity column. The GST-fusion proteins were further purified using a Superdex-75 column (GE Healthcare). Protein quality was checked by SDS-PAGE, and protein concentration was quantified by Pierce BCA assay kits (Thermo). GTP hydrolysis was monitored over time using a malachite green-based colorimetric assay according to the manufacturer’s protocol (Sigma, MAK1113). Purified recombinant proteins at a final concentration of 0.1–0.15 μm were incubated in assay buffer (50 mm Tris, 50 mm NaCl, 4 mm MgAc2, and 0.5 mm EDTA, pH 7.5) with 1 mm GTP for 30 min at 23°C.
Immunofluorescence
The fusion protein was expressed in BL21 CodonPlus cells and purified under native conditions. Purified His-PDV2(1–213aa) was injected into rabbits as an antigen to produce polyclonal antibodies and to purify anti-PDV2 antibody from serum. Three-week-old leaf samples from wild-type plants expressing CFP-DRP5Bg were fixed, embedded, sectioned, and immunoprobed as described previously, with minor modifications (Vitha and Osteryoung, 2011). Rehydrated sections were incubated overnight in a mixture of anti-PDV2 (1:400) and mouse monoclonal anti-GFP (1:1000; JL-8, Clontech) at room temperature after blocking, washed, and incubated in a mixture of Alexa Fluor 488 donkey antimouse and Alexa Fluor 594 goat antirabbit at a dilution of 1:400 (A21202 and A11037, Invitrogen). The samples were mounted onto slides using ProLong Diamond Antifade Mountant (Life Technologies).
Microscopy and Image Analysis
Chloroplast morphology in living cells was observed under a Zeiss Axio Imager M2 microscope equipped with a 40×/0.95 objective and differential interference contrast system, and color images were captured with a Q-imaging MP5 camera. For chloroplast number analysis, fixed leaf tissue was prepared as previously described (Pyke and Leech, 1991) and visualized under a Zeiss Axio Imager M2 microscope equipped with a 40×/0.95 objective. Image analysis was performed using ImageJ (National Institutes of Health). Fluorescent signals from GFP, YFP, CFP, and chlorophyll from living cells and Alexa Fluor 488 or 594 signals from fixed samples were observed under a Leica DM5500 microscope equipped with a 63×/1.4 oil objective and fluorescence filter cubes for CFP, L5, YFP, and TX2, and images were captured with an Andor Zyla monochrome CCD. Time-lapse and FRAP experiments were performed using a Zeiss LSM780 inverted confocal microscope equipped with a 63×/1.4 oil objective and 488-nm laser for GFP and chlorophyll. The emitted fluorescence was collected at 490–550 nm for GFP and 600–700 nm for chlorophyll. Rosette leaves from 3-week-old plants without the lower epidermal layers were directly placed into an imaging dish and sealed with a coverglass with double-sided sticky tape. The confocal aperture was opened to 3 Airy units. In the FRAP experiments, three prebleached images were collected at 2-s intervals. A small region of interest was then bleached at 100% 488-nm laser intensity. The recovery of fluorescence in the photobleached section of the ring structure was recorded in a time-lapse sequence. To achieve good temporal resolution of postbleach recovery and to minimize the overall light dose and phototoxicity by the imaging beam, the postbleaching images were acquired at variable, progressively longer time intervals. The postbleaching imaging sequence consisted of four segments (20 × 5, 20 × 10, 20 × 20, and up to 20 × 30 s). The image stacks and the corresponding time stamps for each frame were collected with Zeiss Zen 2012 software and exported for analysis. As chloroplasts exhibited photophobic movements when irradiated with the imaging laser, only datasets where chloroplasts remained in focus were used. Each raw FRAP dataset was processed to generate a photobleaching-corrected and normalized fluorescence recovery dataset using the FRAPanalyzer program (Halavatyi et al., 2010), and the normalized average FRAP recovery dataset was subjected to curve fitting with Origin 8.0 software (OriginLab). The data were fit to the single-exponential f(t) = A(1 − e−kt) equation. t1/2 was calculated as t1/2 = ln(1/2)/−k. Analysis of statistically significant differences between average recovery half-time and percent recovery of GFP-DRP5Bg in different plants was performed using a two-tailed Student’s t-test. For the FRAP control samples, leaf tissue was fixed with 3.5% (w/v) glutaraldehyde at room temperature for 30 min, and washed with phosphate buffer solution. The tissue was subjected to FRAP analysis, and images were processed using ImageJ and Photoshop software.
Immunoblot Analysis
Each 100-mg sample of fresh leaf tissue from 3-week-old seedlings was ground in liquid nitrogen and combined with 200 μL of 2× SDS loading buffer. The sample (15 μL of protein extract) was loaded onto a 10% SDS-PAGE minigel and transferred onto a supported nitrocellulose membrane (Bio-Rad). The membranes were blocked using 0.2% (v/v) Tris-buffered saline plus Tween 20 solution containing 2% (w/v) milk for 1 h at room temperature and probed overnight with primary antibodies. The following antibodies diluted in blocking solution were used: anti-GFP (1:2,500; JL-8, Clontech), antiactin (1:10,000; A0480, Sigma), and anti-PDV2 (1:2,500). Bound antibodies were detected with peroxidase-coupled secondary antibodies using an enhanced chemiluminescence system.
BiFC Analysis
The BiFC assays were performed as described previously (Chen et al., 2018).
Accession Numbers
Sequence data from this study can be found in The Arabidopsis Information Resource databases under the following accession numbers: At5g53280 (PDV1), At2g16070 (PDV2), At3g19720 (DPR5B), and At5g58140 (PHOT2).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Investigation of the interactions of DRP5B with PDV1 and PDV2 by BiFC assays.
Supplemental Figure S2. Localization patterns of CFP-DRP5Bg in mesophyll cells of wild-type, pdv1-1, and pdv2-1 plants.
Supplemental Table S1. Primers used in this study.
Supplemental Video S1. Fluorescence recovery of the DRP5B ring at the chloroplast division site in mesophyll cells of phot2-1 expressing GFP-DRP5Bg.
Supplemental Video S2. FRAP analysis of mobility of DRP5B in the ring structure at the chloroplast division site in mesophyll cells of drp5B-2 phot2-1 expressing GFP-DRP5B.
Supplemental Video S3. FRAP analysis of mobility of DRP5B(T82D) in the ring structure at the chloroplast constriction site in mesophyll cells of the transgenic drp5B-2 phot2-1.
Supplemental Video S4. Fluorescence recovery of the DRP5B ring on chloroplasts of pdv1-1 phot2-1/GFP-DRP5Bg.
Supplemental Video S5. Fluorescence recovery of the DRP5B ring on chloroplasts of PDV2-1/pdv2-1 phot2-1 expressing GFP-DRP5Bg.
Acknowledgments
We thank Dr. Masamitsu Wada for providing the Arabidopsis phot2-1 mutant and the Arabidopsis Biological Resource Center for another ethyl methanesulfonate-induced and T-DNA insertional mutant. We thank Dr. Katherine Osteryoung for providing valuable comments, Dr. Xian-yong Sheng for help in FRAP analysis using the Zeiss LSM 780, and Dr. Shi Xu for assistance in protein purification.
Footnotes
This work was supported by grants from the National Science Foundation of China (NSFC31470296), the Science and Technology Development Program of Beijing Municipal Commission of Education (KM2018100280011), the Program for Changjiang Scholars and Innovative Research Team in University (IRT-17R75), and Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (IDHT20180518).
References
- Aung K, Hu J (2012) Differential roles of Arabidopsis dynamin-related proteins DRP3A, DRP3B, and DRP5B in organelle division. J Integr Plant Biol 54: 921–931 [DOI] [PubMed] [Google Scholar]
- Chen L, Sun B, Gao W, Zhang QY, Yuan H, Zhang M (2018) MCD1 associates with FtsZ filaments via the membrane-tethering protein ARC6 to guide chloroplast division. Plant Cell 30: 1807–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL (1994) Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol 127: 915–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, De Camilli P (2012) Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 13: 75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MG, Jenni S, Nunnari J (2011) The crystal structure of dynamin. Nature 477: 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Kadirjan-Kalbach D, Froehlich JE, Osteryoung KW (2003) ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery. Proc Natl Acad Sci USA 100: 4328–4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn JM, Froehlich JE, Osteryoung KW (2008) Arabidopsis ARC6 coordinates the division machineries of the inner and outer chloroplast membranes through interaction with PDV2 in the intermembrane space. Plant Cell 20: 2460–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn JM, Yang Y, Vitha S, Schmitz AJ, Hemmes M, Miyagishima SY, Osteryoung KW (2009) PARC6, a novel chloroplast division factor, influences FtsZ assembly and is required for recruitment of PDV1 during chloroplast division in Arabidopsis. Plant J 59: 700–711 [DOI] [PubMed] [Google Scholar]
- Halavatyi AA, Nazarov PV, Al Tanoury Z, Apanasovich VV, Yatskou M, Friederich E (2010) A mathematical model of actin filament turnover for fitting FRAP data. Eur Biophys J 39: 669–677 [DOI] [PubMed] [Google Scholar]
- Holtsmark I, Lee S, Lunde KA, Auestad K, Maple-Grødem J, Møller SG (2013) Plastid division control: The PDV proteins regulate DRP5B dynamin activity. Plant Mol Biol 82: 255–266 [DOI] [PubMed] [Google Scholar]
- Hong Z, Bednarek SY, Blumwald E, Hwang I, Jurgens G, Menzel D, Osteryoung KW, Raikhel NV, Shinozaki K, Tsutsumi N, et al. (2003) A unified nomenclature for Arabidopsis dynamin-related large GTPases based on homology and possible functions. Plant Mol Biol 53: 261–265 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Klockow B, Tichelaar W, Madden DR, Niemann HH, Akiba T, Hirose K, Manstein DJ (2002) The dynamin A ring complex: Molecular organization and nucleotide-dependent conformational changes. EMBO J 21: 240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong SG, Arai Y, Suetsugu N, Yanagida T, Wada M (2013) Rapid severing and motility of chloroplast-actin filaments are required for the chloroplast avoidance response in Arabidopsis. Plant Cell 25: 572–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa T, Kuroiwa H, Sakai A, Takahashi H, Toda K, Itoh R (1998) The division apparatus of plastids and mitochondria. Int Rev Cytol 181: 1–41 [DOI] [PubMed] [Google Scholar]
- Lopez-Juez E, Pyke KA (2005) Plastids unleashed: Their development and their integration in plant development. Int J Dev Biol 49: 557–577 [DOI] [PubMed] [Google Scholar]
- Marks B, Stowell MH, Vallis Y, Mills IG, Gibson A, Hopkins CR, McMahon HT (2001) GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature 410: 231–235 [DOI] [PubMed] [Google Scholar]
- Marrison JL, Rutherford SM, Robertson EJ, Lister C, Dean C, Leech RM (1999) The distinctive roles of five different ARC genes in the chloroplast division process in Arabidopsis. Plant J 18: 651–662 [DOI] [PubMed] [Google Scholar]
- McAndrew RS, Froehlich JE, Vitha S, Stokes KD, Osteryoung KW (2001) Colocalization of plastid division proteins in the chloroplast stromal compartment establishes a new functional relationship between FtsZ1 and FtsZ2 in higher plants. Plant Physiol 127: 1656–1666 [PMC free article] [PubMed] [Google Scholar]
- Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE (2011) Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol 18: 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishima SY, Froehlich JE, Osteryoung KW (2006) PDV1 and PDV2 mediate recruitment of the dynamin-related protein ARC5 to the plastid division site. Plant Cell 18: 2517–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K, Kabeya Y, Suzuki K, Mori T, Ichikawa T, Matsui M, Nakanishi H, Miyagishima SY (2009) The PLASTID DIVISION1 and 2 components of the chloroplast division machinery determine the rate of chloroplast division in land plant cell differentiation. Plant Cell 21: 1769–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung KW, Pyke KA (2014) Division and dynamic morphology of plastids. Annu Rev Plant Biol 65: 443–472 [DOI] [PubMed] [Google Scholar]
- Possingham JV, Lawrence ME (1983) Controls to plastid division In Bourne GF, Danielli F, and Jeon KW, eds, International Review of Cytology, Vol 84 Academic Press, New York, pp 1–56 [Google Scholar]
- Post-Beittenmiller D, Roughan G, Ohlrogge JB (1992) Regulation of plant fatty acid biosynthesis: Analysis of acyl-coenzyme A and acyl-acyl carrier protein substrate pools in spinach and pea chloroplasts. Plant Physiol 100: 923–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Leech RM (1991) Rapid image analysis screening procedure for identifying chloroplast number mutants in mesophyll cells of Arabidopsis thaliana (L.) Heynh. Plant Physiol 96: 1193–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaman A, Elde NC, Turkewitz AP (2008) A dynamin-related protein required for nuclear remodeling in Tetrahymena. Curr Biol 18: 1227–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Schmid SL (2008) Real-time detection reveals that effectors couple dynamin’s GTP-dependent conformational changes to the membrane. EMBO J 27: 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Schmid SL (2018) The dynamin superfamily. Curr Biol 28: R411–R416 [DOI] [PubMed] [Google Scholar]
- Robertson EJ, Rutherford SM, Leech RM (1996) Characterization of chloroplast division using the Arabidopsis mutant arc5. Plant Physiol 112: 149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BD, Leonard M, Schmid SL (2004) Dynamin GTPase domain mutants that differentially affect GTP binding, GTP hydrolysis, and clathrin-mediated endocytosis. J Biol Chem 279: 40431–40436 [DOI] [PubMed] [Google Scholar]
- Stowell MHB, Marks B, Wigge P, McMahon HT (1999) Nucleotide-dependent conformational changes in dynamin: Evidence for a mechanochemical molecular spring. Nat Cell Biol 1: 27–32 [DOI] [PubMed] [Google Scholar]
- Sung MW, Shaik R, TerBush AD, Osteryoung KW, Vitha S, Holzenburg A (2018) The chloroplast division protein ARC6 acts to inhibit disassembly of GDP-bound FtsZ2. J Biol Chem 293: 10692–10706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek AM, Meyerowitz EM (1991) Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351: 411–414 [DOI] [PubMed] [Google Scholar]
- Vitha S, Froehlich JE, Koksharova O, Pyke KA, van Erp H, Osteryoung KW (2003) ARC6 is a J-domain plastid division protein and an evolutionary descendant of the cyanobacterial cell division protein Ftn2. Plant Cell 15: 1918–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitha S, McAndrew RS, Osteryoung KW (2001) FtsZ ring formation at the chloroplast division site in plants. J Cell Biol 153: 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitha S, Osteryoung KW (2011) Immunofluorescence microscopy for localization of Arabidopsis chloroplast proteins In Jarvis R, ed, Chloroplast Research in Arabidopsis Methods in Molecular Biology, Vol 774 Humana Press, Totowa, NJ, pp 33–58 [DOI] [PubMed] [Google Scholar]
- Wang W, Li J, Sun Q, Yu X, Zhang W, Jia N, An C, Li Y, Dong Y, Han F, et al. (2017) Structural insights into the coordination of plastid division by the ARC6-PDV2 complex. Nat Plants 3: 17011. [DOI] [PubMed] [Google Scholar]
- Warnock DE, Hinshaw JE, Schmid SL (1996) Dynamin self-assembly stimulates its GTPase activity. J Biol Chem 271: 22310–22314 [DOI] [PubMed] [Google Scholar]
- Warrens AN, Jones MD, Lechler RI (1997) Splicing by overlap extension by PCR using asymmetric amplification: An improved technique for the generation of hybrid proteins of immunological interest. Gene 186: 29–35 [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Miyagishima SY, Kuroiwa H, Kuroiwa T (2012) The plastid-dividing machinery: Formation, constriction and fission. Curr Opin Plant Biol 15: 714–721 [DOI] [PubMed] [Google Scholar]
- Zhang M, Chen C, Froehlich JE, TerBush AD, Osteryoung KW (2016) Roles of Arabidopsis PARC6 in coordination of the chloroplast division complex and negative regulation of FtsZ assembly. Plant Physiol 170: 250–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Hu J (2010) The Arabidopsis chloroplast division protein DYNAMIN-RELATED PROTEIN5B also mediates peroxisome division. Plant Cell 22: 431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]