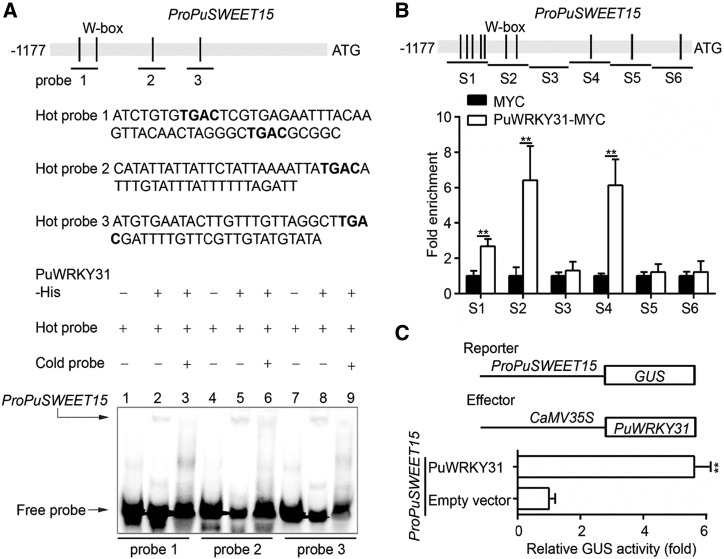
Figure 5.
PuWRKY31 promotes PuSWEET15 transcription. A, EMSA analysis of PuWRKY31 binding to the PuSWEET15 promoter. The hot probe was a biotin-labeled PuSWEET15 promoter, while the cold probe was a nonlabeled competitive probe (with a 100-fold higher concentration than the hot probe). PuWRKY31-His was purified and used for DNA-binding assays. The sequence of the biotin labeled probe is shown and the W-box motif is highlighted in bold. B, ChIP-PCR showing the in vivo binding of PuWRKY31 to the PuSWEET15 promoter. Cross-linked chromatin samples were extracted from PuWRKY31-MYC-overexpressing NG pear fruit and precipitated with an anti-MYC antibody. Eluted DNA was used to amplify the sequences neighboring the W-box by qPCR. Six regions (S1–S6) were analyzed. Fruit overexpressing GFP were used as negative controls. The ChIP assay was repeated three times and the enriched DNA fragments in each ChIP were used as one biological replicate for qPCR. C, Schematic representation of the GUS reporter vector containing the PuSWEET15 promoter and the effector vector containing PuWRKY31. The effector reporter vectors were infiltrated into Nicotiana benthamiana leaves to analyze the regulation of GUS activity. Three independent infiltrations were performed, and the error bars represent the se. Asterisks indicate significant difference as determined by Student’s t test (**P < 0.01).