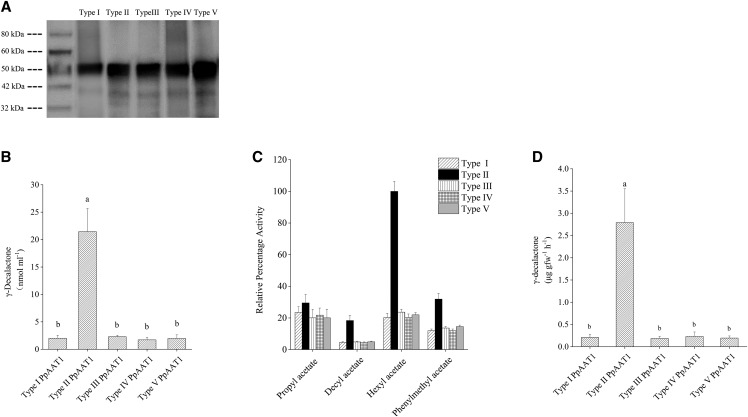
Figure 6.
Enzymatic activity of the five PpAAT1 variants. A, Western analysis of the five PpAAT1 variants. B, The content of γ-decalactone produced by the five kinds of PpAAT1 in vitro. C, Comparison of esterification activity of the five PpAAT1 variants ± se (n = 6). The differences in activity between the type II and other PpAAT1s are significant for all the esters (P ≤ 0.05) except propyl acetate. The 100% relative activity corresponds to 0.22 μmol min–1 mg–1 hexyl acetate. D, γ-Decalactone contents detected in transgenic N. benthamiana leaves expressing the five PpAAT1 variants. Data are presented as means ± se (n = 6), and letters represent significant differences at P ≤ 0.05 as determined using ANOVA followed by Fisher’s lsd test.