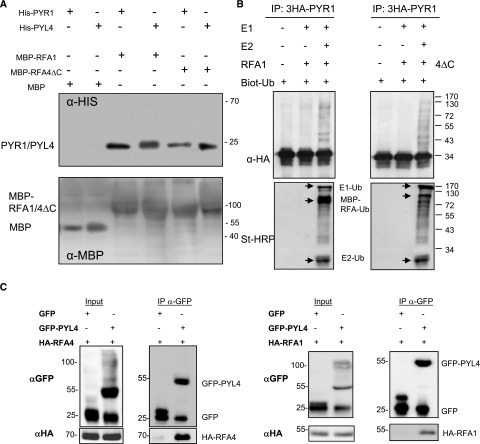
Figure 3.
RFA1 and RFA4 pull down ABA receptors and show E3 Ub ligase activity with PYR1. A, Interaction among PYR1/PYL4 with RFA1 or RFA4ΔC in a pull-down assay. Purified MBP, MBP-RFA1, or MBP-RFA4ΔC (5 μg each) and 5 μg of 6His-PYR1 or PYL4 protein were incubated for 1 h at 4°C with constant rocking in 0.5 mL of binding buffer (20 mm Tris-HCl, pH 7.5, 200 mm NaCl, 1 mm EDTA, and 0.5% [v/v] Tween 20). Next, MBP, MBP-RFA1, or MBP-RFA4DC protein was purified using amylose affinity chromatography, eluted, and analyzed by immunoblotting using anti-His and Ponceau staining. B, MBP-RFA1 or MBP-RFA4DC (2 µg) was assayed for E3 ligase activity in the presence of 100 ng of human E1, 250 ng of purified 6His-AtUBC8 (E2), 3HA-PYR1 immunoprecipitated from an Arabidopsis transgenic line, and 1 µg of biotinylated Ub. After incubation at 30°C for 2 h, the mixture was subjected to SDS-PAGE/blotting followed by detection using either St-HRP or anti-HA-HRP to detect either ubiquitylated or 3HA-tagged proteins, respectively. The components of the ubiquitination cascade (i.e. E1-Ub, E2-Ub, ubiquitinated MBP-RFA1, or MBP-RFA4DC) are indicated by arrows. C, PYL4 coimmunoprecipitates with RFA1 and RFA4. GFP or GFP-PYL4 proteins were expressed in N. benthamiana, and each protein extract was combined with MG132-treated Arabidopsis protein extracts obtained from lines expressing either HA-RFA1 or HA-RFA4. Proteins were immunoprecipitated using anti-GFP and next immunoblotted with anti-HA to detect coimmunoprecipitation of either HA-RFA1 or HA-RFA4.