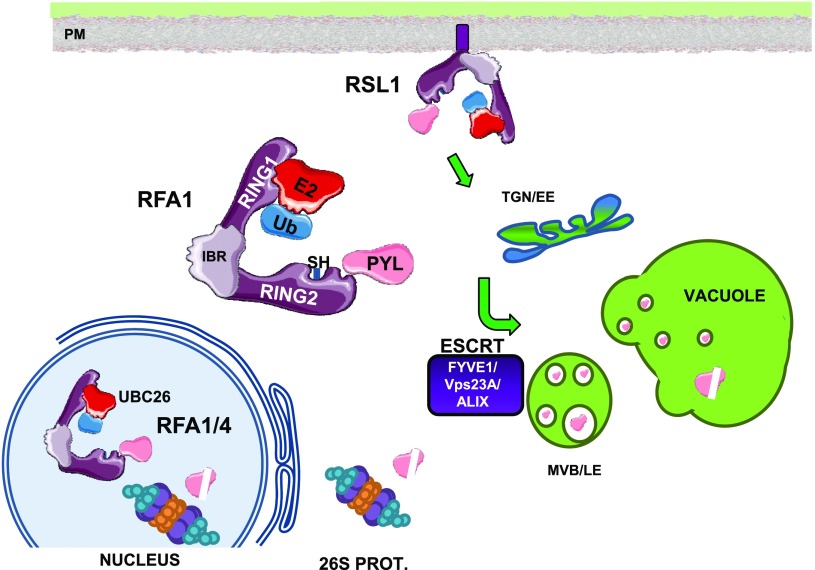
Figure 8.
A working model depicting the targeting of ABA receptors by RSL1, RFA1, or RFA4 at different subcellular locations. Ubiquitination of ABA receptors at the plasma membrane by RSL1 triggers clathrin-mediated endocytosis, transit through the trans-Golgi network/early endosomes (TGN/EE), sorting of ubiquitinated cargo through the ESCRT pathway (involving FYVE1, VPS23A, and ALIX components), delivery to multivesicular bodies/late endosomes (MVB/LE), and finally to the vacuole for degradation. Ubiquitination of receptors at cytosol or nucleus leads to degradation by the 26S proteasome. The UBC26-RFA4-receptor complex regulates the half-life of ABA receptors in the nucleus. The cartoon represents the RING1-IBR-RING2 modular structure of RBR-type E3 ligases and the localization of the active site Cys-SH residue in RING2.